The Epidemiology of Breast Cancer: Incidence and Risk Factors
Celia Byrne
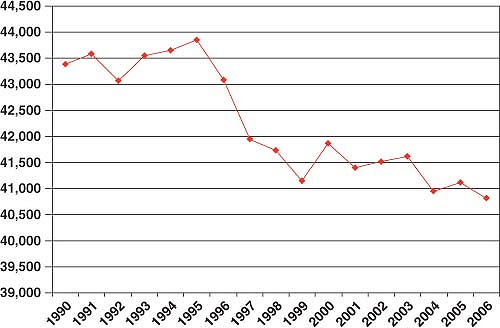
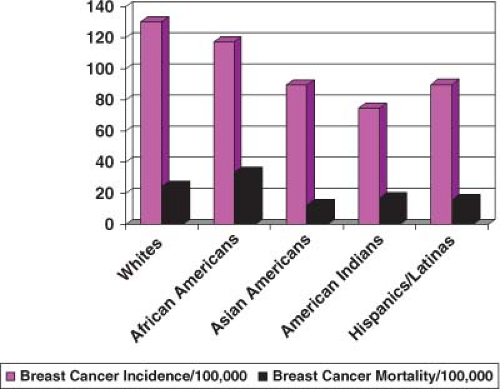
In the United States, breast cancer comprises 27% of all new female cancers, and as such is the most common cancer among women and the second-leading cause of cancer mortality, explaining 15% of female cancer deaths (1). In the United States, an estimated 192,370 women were diagnosed with breast cancer and 40,170 women died from breast cancer in 2009 (1) (Fig. 1.1). Breast cancer incidence and mortality rates vary by race and ethnicity (2) (Fig. 1.2). Clearly breast cancer remains a substantial cause of morbidity and mortality, and there is need for continued efforts to better understand the etiology of the disease, maintain screening efforts, implement prevention strategies, and develop better treatments. It is important to consider what we know about breast cancer etiology already and how that relates to changes in breast cancer incidence and mortality. Our ability to understand greater details about specific breast cancer risk factors has improved in recent years with the published results from various large pooling studies that have combined data from numerous studies and detailed meta-analyses. While results from smaller studies may vary to some degree, combining the data from many studies has provided a better understanding of risk and has also allowed the determination of risk with small changes in exposure levels and in subgroups of women. Most of the studies presented have evaluated risk factors for invasive breast cancer. However, ductal carcinoma in situ (DCIS), a noninvasive disease that is believed to be a precursor lesion for invasive disease if not treated, shares many of the same risk factors. Some studies of breast cancer include invasive and DCIS cases together. Lobular carcinoma in situ seems to be more of a marker of susceptibility rather than a precursor lesion itself, and it does not share as many risk factors.
Risk Factors for Breast Cancer
Gender, Age, and Race/Ethnicity
Gender and age are recognized as the strongest predictors of breast cancer incidence. The incidence in males is approximately 1% of the incidence rate in females (1). In a woman’s lifetime she has on average a probability of 1 in 8 of developing breast cancer by age 85 years. However, she has only a probability of 1 in 208 by age 39 years, a probability of 1 in 26 between ages 40 and 59 years, a probability of 1 in 29 between ages 60 and 69 years, and a probability of 1 in 16 from age 70 years and older (1). In the United States, race and ethnicity are also associated differentially with invasive breast cancer incidence rates. Overall, White women have the highest rate of invasive breast cancer (130.6/100,000), followed by African Americans (117.5/100,000), Hispanics (90.1/100,000), Asian Americans (89.6/100,000), and Native Americans (75.0/100,000) (2). While Whites have the highest incidence of breast cancer overall, that pattern is not consistent for all age groups (3,4). It has been recognized for some time that African American women under the age of 40 years have a higher rate of invasive breast cancer (15.5/100,000) than White women (13.1/100,000), and then after age 40 years White women have a higher rate of invasive breast cancer (281.3/100,000 vs. 239.5/100,000) (3,4). For women under age 30 years the rate in African American women is 1.52 (95% confidence interval [CI] 1.34 to 1.73) times that of White women (3). African Americans have the highest breast cancer mortality rates (33.5/100,000), followed by Whites (24.4/100,000), Native Americans (17.1/100,000), Hispanics (15.8/100,000), and Asians (12.6/100,000) (2).
Early Life Events
Recent studies have suggested that breast cancer risk is impacted by early life events even prenatally, and the early life period in utero, in childhood, and in adolescence may potentially be an important window of risk for breast cancer when breast tissue may be more susceptible to carcinogenic exposures (5,6). Birth weight is positively associated with maternal sex hormones and insulin-like growth factor-1 (IGF-1) levels, both of which are involved in initiation and promotion of breast cancer (7). In a meta-analysis of studies examining early life factors and breast cancer risk, women with higher birth weights had an increased relative risk (RR) of 23% (95% CI 1.13 to 1.34) (8). Studies of birth length have also shown a positive correlation with breast cancer risk, with an overall summary estimate that longer infants have a RR of 1.28 (95% CI 1.11 to 1.48) compared to shorter infants (8). In contrast, offspring of women who developed preeclampsia or eclampsia are at decreased risk of developing breast cancer (9), with a RR of 0.48 (95% CI 0.30 to 0.78) (8). The associations with other measures of in utero exposure, such as being a twin, gestational age, and diethylstilbestrol exposure, are less consistent in associations across studies (5,6,8).
Age At Menarche
It has been long recognized that the younger a woman’s age at menarche, the higher is her risk of developing breast cancer (10). However, a recent study of the participants in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial indicated that the magnitude of the association between age at menarche and breast cancer may not be as strong a predictor of breast cancer as previously considered. Age at menarche has been decreasing over time in the United States and in many developed and developing countries. The factors most influencing age at menarche, such as the prevalence of obesity, also have been changing over time (11).
Age At Menopause
Earlier age at menopause has been associated with reduced risk of breast cancer (10). One theory linked age at menarche and age at menopause, suggesting that the longer a woman was exposed to premenopausal hormone levels (time between menarche and menopause), the greater was her risk. As with age at menarche, age at menopause has also been changing, with later age at menopause being more common (12). In the recent analysis from the PLCO cohort, women with later age at menopause (55+ years) compared to those with early age at menopause (<45 years) had a RR of 1.29 (1.03 to 1.62). Again the magnitude of the association was not as strong an association as anticipated from previous studies (11).
Reproductive History
Age at first birth is another breast cancer risk factor that has been changing over time in developed and developing countries (13). Part of the increase in breast cancer incidence during the 1980s and 1990s can be attributed in part to this trend in delayed childbearing. Compared with women whose first full-term pregnancy was before age 20 years, breast cancer risk increased for women with each year that first full-term pregnancy was delayed. Nulliparous women and those whose first full-term pregnancy was at age 30 years or older typically had a >50% increased risk of breast cancer compared with those whose first full-term birth was before age 20 years (10). Pregnancies that were not full term did not show the protective effect (10). Several studies have now shown a short-term transient increased risk associated with each pregnancy (14,15). Between 5 and 10 years after the pregnancy the protective effect can be seen (14). Given the differentiation that occurs in breast tissue with a full-term pregnancy, it was thought that this process protected against cancer initiation, while in women having a later-age first birth the growth of existing cancer cells would be promoted. Concern was raised whether spontaneous or induced abortion would increase the risk of breast cancer. In an analysis of pooled data from 53 different studies no increased risk was seen with reported spontaneous or induced abortion (16).
Parity
In addition to the effects of age at first birth on reducing breast cancer risk, increased parity is associated with reduced breast cancer risk (10). The protective effect is seen more in older postmenopausal women (10). In an analysis of the Finnish National Population Registry among women born after 1937 who had at least five biological children, short-term spacing between births (<1 year) was associated with an increased risk of breast cancer compared to longer intervals (3+ years) (15). However, unlike the previously reported protective association with increased parity, from studies conducted primarily among White women, a large prospective study in African American women (Black Women’s Health Study) found an increased risk with high parity for women younger than 45 years and a decrease for those 45 years and older (17). These differential associations between parity and breast cancer risk might in part explain the crossover in breast cancer rates seen between African American and White women in the United States.
Breast-Feeding
Results have been inconsistent from studies examining whether there is a separate protective effect with breast-feeding and breast cancer risk. Given the reduction in breast-feeding practices in the United States during most of the 20th century it is now believed that many of these studies lacked sufficient exposure prevalence to be able to evaluate the separate effects of breast-feeding from age at first birth and parity (10). In a reanalysis of 47 studies from 30 countries with data on 50,302 breast cancer cases and 96,973 controls, the relative risk for breast cancer decreased 4.3% for each 12 months of breast-feeding in addition to a 7.0% decrease for each birth (18). These researchers concluded that the longer a woman breast-fed, the more she reduced her breast cancer risk, and that the lack of long-duration breast-feeding in the developed countries contributed to the higher rates of breast cancer (18). Thus, in addition to the health benefits that breast-feeding holds for the infant, women may help to lower their breast cancer risk by maintaining breast-feeding for longer durations.
Family History of Breast Cancer
Having a family history of breast cancer has been recognized as a risk factor for breast cancer that reflects both shared genetic and shared environmental exposures within a family. While this is the breast cancer risk factor that is most recognized by the public, only about 11% of all breast cancer cases have a family history of the disease (19). Having any first-degree family history (mother or sister with breast cancer) is associated with an approximate twofold increase in breast cancer risk. The risk increases with the number of affected relatives, with relative risks of 1.80 (95% CI 1.69 to 1.91), 2.93 (95% CI 2.36 to 3.64), and 3.90 (95% CI 2.03 to 7.49) with one, two, and three or more affected relatives, respectively (19). These strong associations with a multiplex family history provided the background for the search for specific genes within families that might explain the risk (see description of genetic risk factors).
Migratory Studies
Breast cancer rates vary quite dramatically among different countries across the world. Furthermore, as individuals migrate from low-risk countries to high-risk countries their risk begins to increase within one or two generations (20). Breast cancer rates increased among women migrating to the United States from Asian countries with low national rates within one or two generations (20). These differences in rates between countries that would change with migration led to the speculation that environmental influences—particularly dietary factors—would be related to breast cancer risk. Stemming from correlation studies of dietary fat consumption in those countries and breast cancer rates, the leading theory is that dietary fat intake must be driving the increased breast cancer rates in the developed countries. As countries become more developed, their breast cancer rates often rise, becoming closer to that of the United States and Western Europe.
Nutritional Factors
A large pooling study of the effects of fat on breast cancer risk found no association between breast cancer and risk for total fat (pooled RR of 1.00; 95% CI 0.98 to 1.03) or most subtypes of fat, other than a weak association with saturated fat intake (21). However, these studies were conducted primarily among postmenopausal women and had minimal data on dietary intake early in adult life. More recently, analysis of a large cohort of postmenopausal women in the NIH-AARP Diet and Health Study suggested that there may be a modest association between a twofold increase in percent energy from total fat and breast cancer, with a relative risk of 1.32 (95% CI 1.11 to 1.58) when corrected for measurement error (22). In a study of premenopausal women in the Nurses’ Health Study II (NHSII) a modest association was found between intake of animal fat and increased breast cancer risk, with a RR of 1.0, 1.28 (95% CI 1.00 to 1.64), 1.37 (95% CI 1.07 to 1.75), 1.54 (95% CI 1.20 to 1.97), and 1.33 (95% CI 1.02 to 1.73) for each quintile of increasing animal fat intake. It could be that the modest associations seen in these two studies are real and they were more evident due to the sufficiently wide range in exposures (40% of calories from fat compared to 20% of calories from fat) in the NIH-AARP study and the evaluation of premenopausal women from the NHS II cohort who had exposures sufficiently early enough during their life time to impact their breast cancer risk. The Women’s Health Initiative Dietary Modification Trial found a 9% reduction in breast cancer rates among women in the low-fat dietary pattern group compared to the control group after 8.1 years of follow-up. This modest association was also accompanied by a lower total caloric intake, lower body weight, and increased fruits and vegetables, fiber, and folate compared to the control group. So it is difficult to attribute the change in rate to a change in dietary fat alone (23). While the association between fat and breast cancer is still controversial, it is prudent for a broad range of other health reasons to consume a diet low in calories, low in fat, and high in whole grains, with lots of fruits and vegetables, which might result in weight loss.
Alcohol
Numerous studies have now reported a modest increased risk of breast cancer with moderate alcohol consumption. A pooled analysis of prospective studies provided sufficient numbers of cases to be able to evaluate types of alcohol to see if effects differed and by quantity of alcohol consumed (24). Risk did not vary by type of alcohol, and for each 10 g/d of alcohol intake breast cancer risk increased by 1.09 (95% CI 1.04 to 1.13) (24). In a reanalysis of 53 studies the relative risk of breast cancer was 7.1% (95% CI 5.5% to 8.7%; p < 0.00001) for each additional 10 g/d of alcohol consumed (25). Despite the fairly consistent associations between alcohol intake and increased breast cancer risk, it is not clear how alcohol intake increases risk. Alcohol intake has been reported to change circulating hormone levels, and this could be how it increases breast cancer risk, or the alcohol metabolites may be carcinogenic (26). Several studies (27) have found that among women with a high dietary folate intake alcohol was not associated with increased breast cancer risk, yet other studies have not seen this interactive effect between dietary folate and alcohol intake (28,29). It seems that among women who consume alcohol, reducing alcohol consumption could potentially help to reduce their risk of breast cancer.
Smoking
Whether smoking has an effect on breast cancer risk has been quite controversial. Smoking has been associated with lower circulating estrogens and earlier age at menopause, two
exposures that might reduce the risk of breast cancer. In light of the inconsistent findings, some have asked whether exposure must occur early in a woman’s life, perhaps prior to her first pregnancy, to have an impact. The analysis in the California Teacher’s Study found an increased hazard ratio of 1.32 (95% CI 1.10 to 1.57) for current smokers compared to never smokers (30). In addition to active smoking, many consider passive smoking to be just as potentially harmful. Analysis of the Million Women’s Study and a meta-analysis found no association between passive smoking and breast cancer risk (31). The reanalysis of the data from the 53 epidemiologic studies indicated that smoking was associated with breast cancer but only among those who consumed alcohol (25). Most likely, both active and/or passive smoking are associated with increased risk of breast cancer for some women. We do not understand which women are susceptible to having smoking increase their risk of breast cancer. Smoking is associated with a multitude of other health risks, so there is no reason for a physician to wait to learn about breast cancer risk to counsel all patients regarding the need to quit smoking.
exposures that might reduce the risk of breast cancer. In light of the inconsistent findings, some have asked whether exposure must occur early in a woman’s life, perhaps prior to her first pregnancy, to have an impact. The analysis in the California Teacher’s Study found an increased hazard ratio of 1.32 (95% CI 1.10 to 1.57) for current smokers compared to never smokers (30). In addition to active smoking, many consider passive smoking to be just as potentially harmful. Analysis of the Million Women’s Study and a meta-analysis found no association between passive smoking and breast cancer risk (31). The reanalysis of the data from the 53 epidemiologic studies indicated that smoking was associated with breast cancer but only among those who consumed alcohol (25). Most likely, both active and/or passive smoking are associated with increased risk of breast cancer for some women. We do not understand which women are susceptible to having smoking increase their risk of breast cancer. Smoking is associated with a multitude of other health risks, so there is no reason for a physician to wait to learn about breast cancer risk to counsel all patients regarding the need to quit smoking.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree