CHAPTER 14 The Diagnostic Approach to the Patient with Proptosis
Introduction
• Learn to recognize the patient with proptosis
• Learn the approach to taking a basic history and performing a physical examination
• Know when imaging is necessary and what imaging studies are best
• Be able to develop a differential diagnosis based on the history and physical examination and imaging studies
• Become familiar with common orbital problems in adults and children
The normal anatomy and examination of the orbit
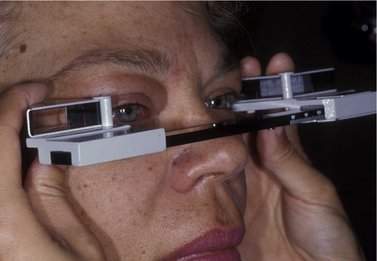
Hertel measurements
An exophthalmometer is used to measure the prominence of the eye. The most common exophthalmometer used is the Hertel exophthalmometer (Figure 14-1). The Hertel exophthalmometer measures the anterior projection of the eye, from the lateral orbital rim to the cornea (Table 14-1). When you use the Hertel exophthalmometer, be sure to lightly push the instrument against the lateral orbital rim and make the width or base setting of the exophthalmometer as narrow as comfortable for the patient. Try to use the same base setting for an individual patient each time you measure the prominence of the eye. Remember that measurements with the Hertel exophthalmometer are not exact, but you should be able to obtain repeatable measurements within 1–2 mm. When you learn to use the Hertel exophthalmometer, compare your readings with those of a more experienced examiner so that you can make sure you are measuring correctly. As the Hertel exophthalmometer uses the lateral orbital rim at a reference point, any surgery, disease, or trauma that changes the position of the lateral rim will affect the Hertel measurements. In cases where the lateral rim is not in normal position, you can use an exophthalmometer designed by Thomas Naugle. This device uses the forehead and cheek as reference points.
Table 14-1 Average Hertel measurements
| Race | Hertel measurement (mm) |
|---|---|
| Asian | 18 |
| White | 20 |
| African American | 22 |
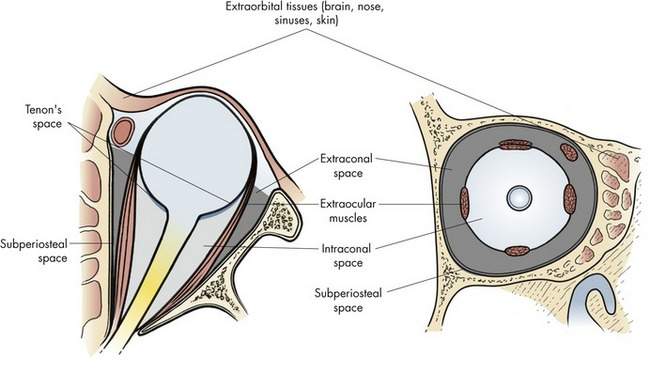
The surgical spaces of the orbit
The orbit is conceptually and anatomically divided into surgical spaces (Box 14-1).
The intraconal space, sometimes called the central surgical space, contains the optic nerve and orbital fat (Figure 14-2). Many tumors arise within the intraconal space or push their way into this space. The most widely discussed tumors of the orbit, optic nerve glioma and optic nerve meningioma, occur in the intraconal space.
The extraorbital space, or periocular tissue, includes all the structures surrounding the orbit: bone, brain, sinuses, nasal, skin, and conjunctiva. A variety of problems originate in these tissues and involve the orbit secondarily. We will talk about some of these conditions at the end of this chapter (Table 14-2).
Table 14-2 Differential diagnosis based on direction of globe displacement in adults
| Displacement | Etiology |
|---|---|
| (1) Axial displacement | |
| Enlarged extraocular muscles | Thyroid orbitopathy |
| Intraconal mass | Cavernous hemangioma |
| Optic nerve tumor | Optic nerve meningioma |
| (2) Nonaxial displacement | |
| (3) Enophthalmos | Scirrhous carcinoma of the breast |
History
The “P’s” of the orbital history and physical examination
We will see in a few paragraphs that we should add a seventh P to the list, past medical history.
History as a clue to the pathologic process
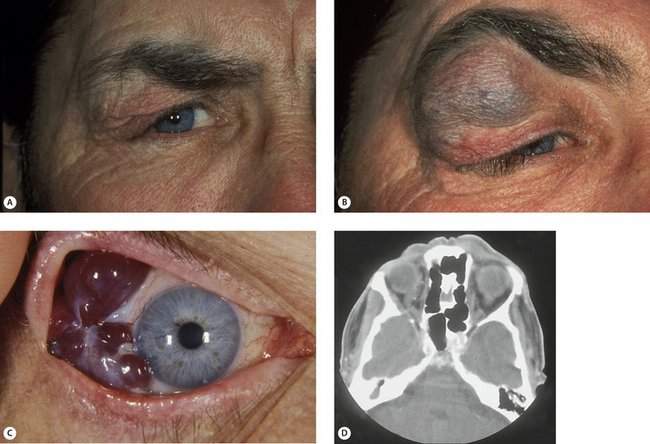
A sudden onset with rapid progression over minutes suggests a hemorrhage (Figure 14-3). Acute processes occurring over hours to days suggest inflammation or infection. Slower processes occurring over weeks to months suggest more chronic types of inflammatory processes such as thyroid disease. Chronic conditions with a vague onset and slow progression over months suggest a benign neoplasm or lymphoma.
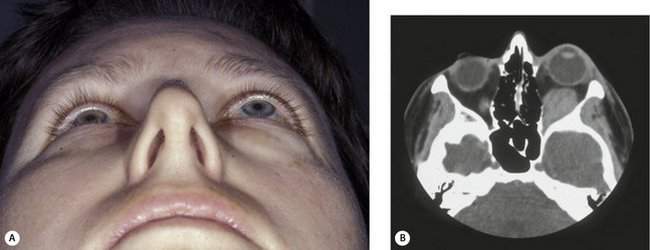
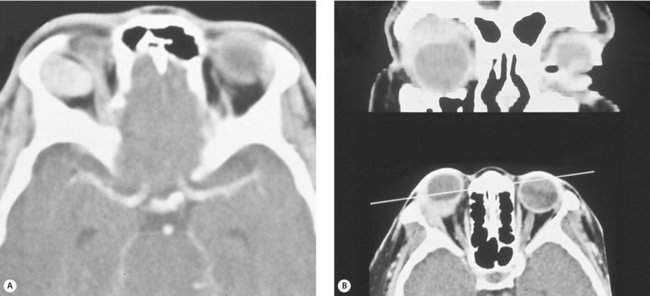
Figure 14-3 Spontaneous orbital hemorrhage. (A) Pain and proptosis developed over minutes with no progression after the initial presentation. (B) CT scan demonstrates a well-circumscribed mass (based on axial and coronal cuts). Drainage of the hematoma was required because of pain (see Figure 15-12). No etiology was determined.
Physical examination of the orbit
Proptosis
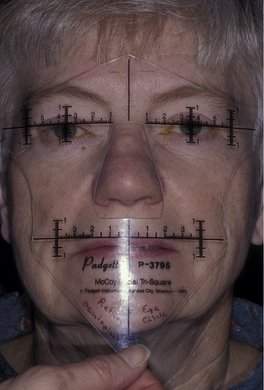
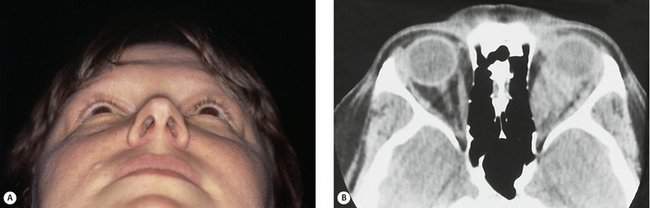
In most cases, when we talk about proptosis, we are really talking about proptosis or an axial displacement of the eye in an anterior direction. When you see axial proptosis, think of thyroid orbitopathy with enlargement of the extraocular muscles. Other intraconal disorders such as optic nerve tumors or a benign cavernous hemangioma may occur within that muscle cone as well and cause axial anterior displacement of the eye. In some conditions, you will see nonaxial displacement of the eye. If you see the eye pushed downward, think of problems arising in the area of the lacrimal gland or, less commonly, defects in the orbital roof due to trauma, encephalocele, or frontal sinus mucocele formation. When you see the eye displaced laterally, there is usually a problem in the ethmoid sinus. The most common situation that displaces the eye laterally is a subperiosteal abscess (an acute process) arising in the ethmoid sinus and extending into the subperiosteal space. Rarely, sinus carcinomas (a slowly progressive process) or mucoceles (a very slowly progressive process) of the ethmoid sinus can cause this type of lateral displacement. You will rarely see the eye being displaced upward. A number of rare conditions can cause this (Figure 14-4). Although lymphoid lesions occur most commonly in the superior orbit, lymphoid lesions are so common that they are the most common cause of an inferior orbital mass. Rarely, tumors arising from the maxillary sinus can erode through the orbital floor and push the eye upward. Likewise, it is rare to see the globe pushed medially. If medial globe displacement is present, the eye usually is also being pushed downward by an enlarged lacrimal gland. You can estimate the nonaxial displacement of the eye with a ruler or use an instrument designed for this purpose known as the McCoy Tri-Square (P-3795, Jarit Instruments, http://www.jarit.com) (Figure 14-5).
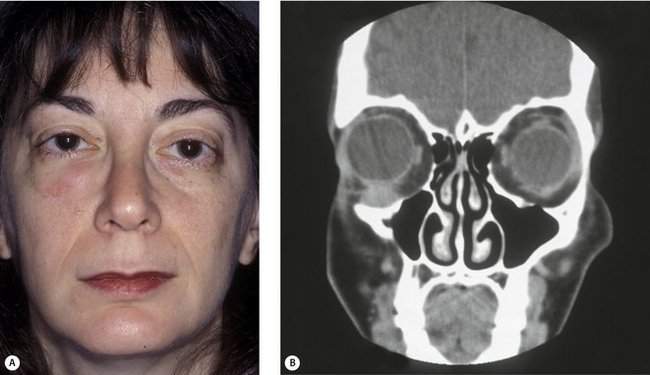
There is an exception to the rule that an orbital mass pushes the eye away from the mass. Scirrhous carcinoma of the breast is an infiltrative sclerosing tumor, which may actually cause an enophthalmos of the eye. You have already asked about past medical history of other carcinomas, so if you heard that the patient has a history of breast carcinoma and you note that eye is sunken, think of metastatic breast cancer (Figure 14-6).
Pulsation
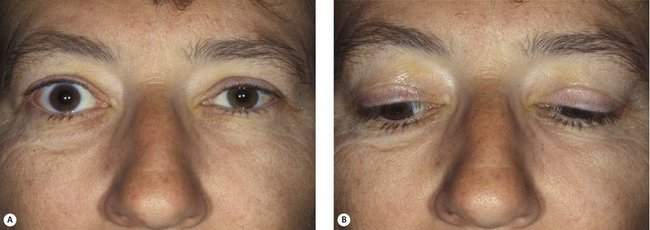
Orbital arterial vascular lesions can pulsate. If the flow is high, you may be able to hear a bruit or feel a thrill. These lesions are rare also. Vascular abnormalities that are primarily venous are more common than arterial lesions. Venous lesions do not pulsate, but they will usually show enlargement with the Valsalva maneuver or with the head in a dependent position (Figure 14-7).
Periocular changes
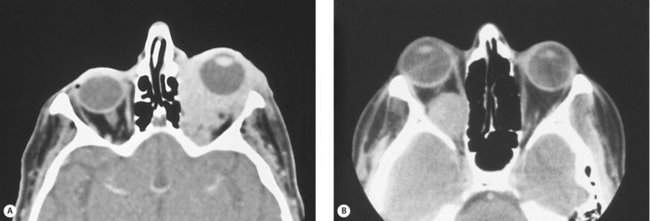
The last point to note in the orbital examination is periocular changes. These include a variety of abnormalities in the skin, conjunctiva, eye, or surrounding periocular tissues. Some periocular changes that are most useful for diagnosis are the temporal flare of the lateral portion of the upper lid and lid lag seen on downgaze in patients with thyroid orbitopathy (Figure 14-8). Other examples of periocular changes include a conjunctival salmon patch suggesting orbital lymphoma (see Figure 14-18), fullness of the temple suggesting a sphenoid wing meningioma (see Figure 14-22), and periocular skin malignancy suggesting intraorbital spread of cutaneous carcinoma.
• You will start by evaluating the change in the position of the eye in terms of axial and nonaxial displacement, suggesting where a mass might be present and pushing the eye away
• Next you will palpate the orbital rims and soft tissues to see if any abnormality is present
• Then you will check briefly for any pulsations
• Last, you will search for other clues in the periocular area that may give you information to develop a differential diagnosis
Orbital imaging
Magnetic resonance imaging
• You can recognize a T1-weighted scan because the vitreous is dark
• You can recognize a T2-weighted scan because the vitreous is white (“bright”)
• Because the density of the signal depends on the density of protons in the tissue, edema (water, i.e., protons) causes a bright signal on the T2-weighted image
• High vascular flow, such as that in the carotid artery, generates no signal (dark), “a flow void,” because the protons are moving too quickly to be imaged
• The protons in bone are too tightly bound to generate a signal, so cortical bone is dark on MRI scans. Marrow spaces will generate a signal
• Look at the T1-weighted scan for the best anatomic detail
• T2-weighted scans show water as “bright,” emphasizing edema or other fluid within a mass
• As you get more familiar with specific disease processes, you can learn the individual characteristics of the T1 and T2 sequences for each process or tumor (don’t worry about that now)
• Look at the fat suppression sequence (the fat is dark) on gadolinium-enhanced scans. Enhancement implies a richly vascularized tumor or inflammation (such as sarcoid, often not visible without enhancement)
Special imaging studies
CT studies for stereotactic navigation are commonly obtained by our ear, nose, and throat (ENT) and neurosurgical colleagues, especially when performing endoscopic operations. You are probably familiar with studies but, if not, you should see the technique in action. By linking the preoperative high resolution images with cameras in the operating room that sense the position of your instruments, you can have real time localization of your position in the patient. This technique is especially useful where the “normal” anatomy is quite variable (paranasal sinuses) and for reoperations where normal landmarks have been altered. It can be helpful for you when you are operating in less familiar areas. For example, I used this when I was less experienced in skull base procedures. CT scanning before and during Valsalva maneuver is helpful for detecting the venous flood seen in orbital varies. 3D CT scans produce amazing pictures (see Figure 14-9). These images are most useful for craniofacial anomalies and extensive facial trauma. 3D scans are valuable for planning a reconstructive operation and are also useful for teaching residents and patients. CTA and MRA are easy ways to view the blood supply of a tumor. For a number of reasons, MRA is usually the first choice for our purposes. Arteriography remains the gold standard for vascular imaging. At the same time, therapeutic selective occlusion of feeding vessels can cure or decrease vascular flow decreasing or eliminating the problem or, in some cases, making operation safer. Similarly, direct venous puncture and occlusion can be helpful in selected cases of varix or other mixed venous malformations. If your practice includes these patients, you will need a strong working relationship with a neurointerventional radiologist. Echography has been used in the imaging of ocular and orbital diseases for many years. In the hands of experienced practitioners, useful information can be obtained. In most centers, CT and MRI have replaced echography in the study of orbital disease.
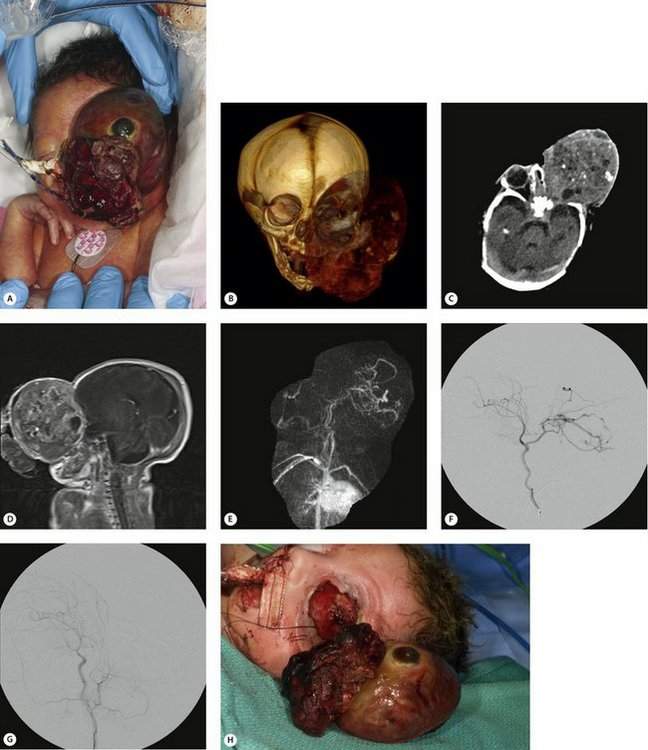
Figure 14-9 Orbital teratoma. (A) Extremely rare and large congenital orbital mass in premature infant. (B) 3D CT reconstruction demonstrates soft tissue mass and large bony orbit. (C) Axial CT scan of head, soft tissue window—shows a large heterogeneous mass in the orbit without extension into the brain. Eye appears to be flattened at the most anterior portion of the mass. (D) Sagittal T1 MRI of the head, gadolinium enhanced, shows heterogeneity prompting vascular studies. (E) MR angiogram (MRA) demonstrates prominent vascular flow from the internal carotid artery branches. (F) Carotid angiography confirmed carotid artery flow. (G) Carotid angiography after occlusion of feeding arterial supply. (H) Successful tumor excision (orbital exenteration) with minimal blood loss.
The information that is available with current imaging techniques and the expertise of our radiology colleagues is incredible. This information can be extremely valuable for diagnosis and surgical planning. A good example is Figure 14-9 which focused our attention on the blood supply of a large congenital mass in a newborn. Vascular studies and embolization made the tumor removal safe for this premature baby weighing only 3 pounds.
Interpretation of orbital imaging
Goals of imaging
• Providing diagnostic information (I want to know what the mass is)
• Providing information used to plan orbitotomy (I want to know the best surgical approach to biopsy the mass)
Location
Position of the mass: the surgical spaces of the orbit
If you cannot tell whether the mass is derived from a normal structure, you should localize the mass within a specific orbital space and describe its position relative to normal structures within the space. We discussed the surgical spaces of the orbit (see Figure 14-2) earlier in this chapter under “The Normal Anatomy and Examination of the Orbit.” The surgical spaces of the orbit are:
Knowledge of the surgical space containing the orbital mass is useful for developing a differential diagnosis and choosing the surgical approach for biopsy. The spatial relationship to the optic nerve and the anterior–posterior position within the intraconal space are especially important in choosing the orbitotomy approach. When you operate in the intraconal space, choose a surgical approach so that you do not cross the optic nerve. Approach medial intraconal masses in the anterior portion of the orbit from a medial anterior orbitotomy. Approach lateral intraconal masses in the anterior orbit from a lateral orbitotomy. Masses arising deep in the intraconal space must be approached transcranially. You can see that the location of a mass in the orbit is critical for the diagnosis and biopsy of the mass. We will discuss the surgical approaches to the orbit in the next chapter.
Imaging clues to the biologic behavior of the mass
Relationship to adjacent soft tissue—a pusher or an eater?
This is important. Does the mass push the adjacent structures aside or does it infiltrate the adjacent tissues? Infiltrative lesions are usually malignant. Well-circumscribed lesions with smooth borders are usually benign (Figure 14-10). Think of these lesions as “pushers” or “eaters.” Pushers are more likely benign. Eaters are more likely malignant. Is the mass pushing against the optic nerve or growing into the optic nerve (Figure 14-11)? The latter lesion is more likely malignant. The relationship to adjacent structures, or borders, gives you a “snapshot in time” estimate of the biologic behavior of the mass. This concept is simple but extremely important.
Relationship to adjacent bone fossa formation or bone erosion
The relationship of a soft tissue mass to the adjacent bone gives similar information about the biologic behavior of the mass. Slow-growing benign masses “push” the bone or cause fossa formation. Aggressive malignant tumors “eat” the bone or cause bone erosion. When you evaluate a lacrimal gland mass, the presence of fossa formation is typical of a benign slow-growing mixed tumor of the lacrimal gland (Figure 14-12).