Chapter 15 Surgical Approaches to the Orbit*
Introduction
Because you are likely to be doing these procedures, I will describe them in detail.
Deep tumors in the orbit are more difficult to expose. Deeper tumors in the medial aspect of the orbit are especially difficult to reach. Specialized anterior orbitotomy techniques can be used to approach deeper tumors; however, you may not want to perform them until you master the more basic procedures. Because these procedures are used less often, I have included less detail for you. Nevertheless, you should know that they exist.
Choosing the surgical approach
There are several factors to consider in choosing the surgical approach:
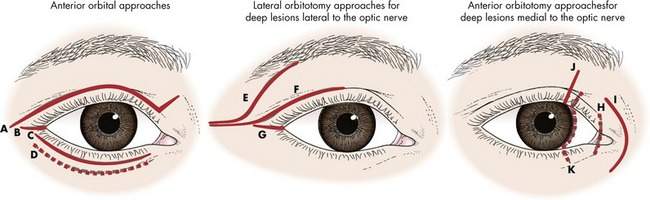
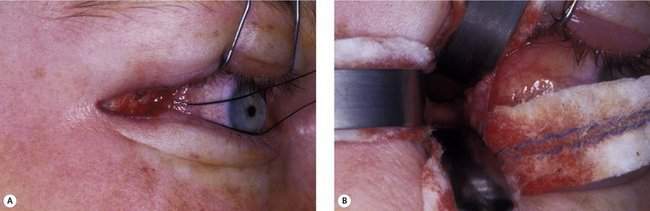
Based on these factors, the safest and most practical approach to reaching the orbital tumor is chosen. In most patients, the skin incision will be chosen to provide optimal scar camouflage by placing it in a skin crease, hiding it on a posterior surface of the eyelid, or placing it adjacent to prominent anatomic landmarks such as the eyebrow or eyelashes (Figure 15-1).
Relationship to the optic nerve
A fundamental principle of orbital surgery is:
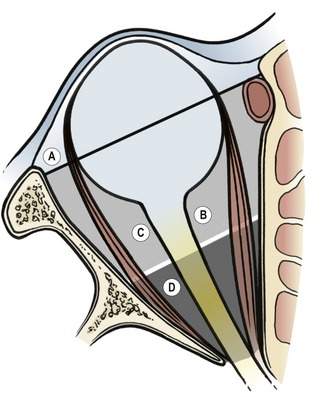
Deep tumors medial to the optic nerve are more difficult to reach because there is no bone that can be easily removed to provide good exposure and access. The surgical spaces of the medial orbit can be reached through deep medial orbitotomy approaches, through either the eyelid or the conjunctiva. Access to the apical medial orbit from these anterior orbitotomy approaches is limited. In unusual situations, the medial orbital wall may be removed and limited access to the deep medial orbit can be obtained (Figure 15-2).
Type of biopsy
The surgical approach may be influenced by the goal of the operation. It is easier to perform an incisional biopsy through a small incision than it is to perform an excisional biopsy. For example, an incisional biopsy of the optic nerve can be performed through either a transconjunctival anterior orbitotomy or a lateral orbitotomy approach. Excision of the entire optic nerve, however, requires a much wider area of exposure, usually provided only by a transcranial orbitotomy approach. You can see that the intent of the operation is a factor in your choice of orbitotomy approach (Box 15-1).
Box 15-1 Surgical Approaches to the Orbit
| Anterior to the equator of the eye | Anterior orbitotomy |
| Posterior to the globe and lateral to the nerve | Lateral orbitotomy |
| Posterior to the globe and medial to the nerve | Deep medial anterior orbitotomy |
| Posterior one third of orbit, optic canal, chiasm | Transcranial orbitotomy |
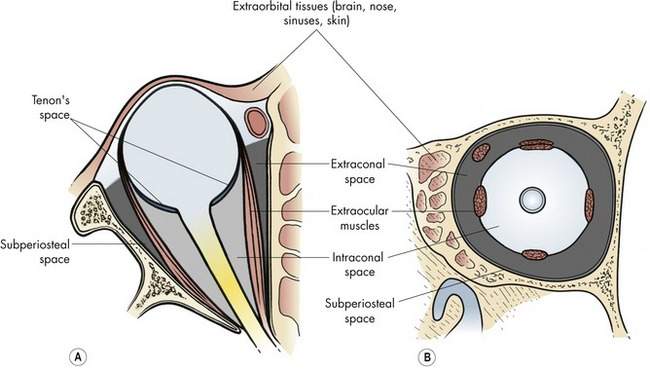
The surgical spaces of the orbit
You will recall that the surgical spaces already discussed in Chapters 2 and 13 are (Figure 15-3):
You should already know these spaces. This section is intended as an illustration of how you will begin to think of the spaces of the orbit and their relationship to the orbitotomy approach. Don’t memorize the specific pathologic processes and approaches mentioned here. We will talk about them again later in this chapter. Just start to get an idea of how you are going to choose the orbitotomy approach, based on the position of the pathologic process and what you have already learned in this chapter.
The extraorbital space includes all the tissues surrounding the orbit: bone, brain, sinus, nasal, skin, and conjunctiva. You are already familiar with some of the many problems that originate in these tissues and involve the orbit secondarily. The surgical approach to many of these tissues is obvious, whereas others involve areas of overlap with other surgical specialties for which interdisciplinary cooperation is essential to the success of the operation.
Names of orbitotomy approaches
• Anterior orbitotomy means that the approach is from the front of the orbit, usually through the eyelid or conjunctiva. In general, an anterior orbitotomy approach does not involve bone resection.
• Lateral orbitotomy means that the approach is from the lateral side of the orbit. In general, the term lateral orbitotomy implies that the lateral rim will be removed. We will see later that a lateral orbitotomy can be performed through a small skin incision at the lateral canthus without any bone removal.
• The terms anterior and deep are opposite. The term superficial orbital tumor is not used. Anterior tumors are palpable and accessible by the “anterior” approaches. Deep is usually used to describe posterior tumors in the orbit.
• Anatomy accessible only via transcranial orbitotomy includes the posterior one third of the orbit, superior orbital fissure, sphenoid wing, and chiasm.
• Deep tumors are posterior to the globe. I use the term deep medial anterior orbitotomy for approaches to intraconal tumors medial to the nerve. As we discussed above, the lateral orbitotomy is used for approaches to deep tumors lateral to the nerve.
• Apical implies the posterior one third of the orbit. I use the term orbitocranial to describe tumors involving the orbital apex and optic canal, chiasm, superior orbital fissure, or other intracranial structures. The transcranial orbitotomy is used to approach apical or orbitocranial tumors. The terms superotemporal orbitotomy and panoramic orbitotomy are sometimes used interchangeably with the term transcranial orbitotomy.
Intraoperative considerations
Orbital instruments
Specialized orbital instruments are used (Box 15-2). Retraction of skin is necessary, using small (Storz double-fixation forceps) or large (Joseph) skin hooks and suture retractors (4-0 silk). Retraction of the orbital fat is facilitated with Sewall and malleable ribbon retractors of various lengths. Neurosurgical cottonoids placed under the retractors prevent fat prolapse into the surgical wound. A variety of periosteal elevators should be available, including Freer, Joseph, and Dean elevators. Bone removal equipment including a power saw, drill, and bone rongeurs are necessary if deep orbitotomy procedures with bone removal are anticipated. A microplating system is useful to repair complex orbital bone cuts. A Freer elevator or a long cotton-tipped applicator is a useful orbital dissection tool. Small neurosurgical dissectors can be helpful.
Box 15-2 Instruments of Special Interest for Orbital Surgery
Bone instruments
Forceps
• Gruenwald nasal dressing forceps, delicate bayonet (Storz N2862)
• Storz ear forceps (alligator type) (Storz X0240)
• Storz sinus cup forceps: small cup biopsy forceps, useful for biopsies and retraction of friable tumor tissue (Storz N2898)
• Takahashi nasal forceps: ethmoidectomy forceps (Storz N2997)
• Hartman–Herzfeld 3 mm cup forceps: small upbiting angled forceps (Storz N0965)
• Wilde nasal forceps for ethmoidectomy procedures
As we discussed in Chapter 1, scissors and forceps for deeper orbital procedures are usually longer than eye instruments for routine procedures. Bayonet-type handles allow comfortable hand position without blocking visualization in deep surgical wounds, especially when an operating microscope is used. Yasargil neurosurgical scissors (which look like long-handled Westcott scissors) with curved or straight blades are good for deep dissections. Westcott scissors are used in anterior orbitotomy procedures. Most eyelid forceps (Paufique forceps) work well for anterior orbital approaches. Myringotomy forceps or any of a variety of small cup biopsy forceps are useful for grasping tissue in deep or tight spaces. The cup forceps or a small “nasal bead” forceps (Hartman–Herzfeld 3 mm cup forceps) is useful for small biopsies of friable tumor tissue. A variety of cautery forceps are helpful, especially microbayonet bipolar forceps (Fischer or Yasargil bipolar forceps) for deep procedures.
Illumination and magnification
Magnification using surgical loupes is standard for anterior orbitotomy procedures. Loupes (2.5 power) from Designs for Vision (Ronkonkoma, NY) are expensive, but great. For deep orbitotomy procedures, you should use the operating microscope for magnification and illumination. The optimal microscope for orbital surgery is mounted on a counter-weighted stand that allows the microscope to be positioned in any direction in three-dimensional space. These stands are commonly used by your neurosurgical colleagues. The gold standard is operating microscopes made by Zeiss. A variety of configurations are available, but opposing microscope heads are ideal for lateral orbitotomy procedures. A 300 mm objective lens provides an adequate distance between the patient and the microscope to move the longer orbital instruments in and out of the operating field without hitting the microscope. An attached video camera and monitor are important for keeping the operating room staff involved.
Exposure and intraorbital dissection
Remember that, as you dissect deeper into the orbit, you should make the deep portions of the surgical wound at least as wide, or wider, than the initial skin incision (make the wound A shaped rather than V shaped). Use a hand-over-hand dissection technique with the orbital retractors to move into deeper orbital tissues (Figure 15-4). As with dissections elsewhere, the key to an effective dissection is to gently spread or pull the involved tissues apart. When you get to a point where you cannot pull the layers apart with the Sewall or malleable retractors, ask your assistant to hold the retractors, keeping the tissue on stretch. You can then use additional blunt dissection with the Freer elevator or cotton-tipped applicator. You can use cautious sharp dissection with a Westcott or Yasargil scissors to open the connective tissue planes of the orbit if blunt dissection through a plane of tissue is too difficult. When the plane is open, use the hand-over-hand technique with the retractors until you reach the structure you are looking for. If you get lost, put your finger in the wound and reorient yourself. It is easy to pass by a smaller lesion.
No doubt there will be times when you are very close, but you cannot see what you are looking for. Palpation can be very helpful for showing you where you are in relation to where you want to be. Once you have identified the mass, gently place dampened neurosurgical cottonoids (1 inch by 3 inches) into the wound using a bayonet forceps. Reposition the retractor over the cottonoid to push the fat behind the retractor and to prevent the orbital fat from prolapsing around the retractor. You may remember the analogous general surgical technique of packing the bowel off with a lap sponge to allow exposure of the abdominal surgical wound. Repeat this using three or four cottonoids to expose the wound (Figure 15-5). With the cottonoids in place, you can remove or reposition the retractors without the surgical wound collapsing on itself.
• Use your finger to palpate the optic nerve and the back of the eye. Note the orientation of your finger
• Strum the optic nerve with the Sewall retractors
• Keep the retractors against the back of the eye as you dissect into the orbit toward the optic nerve
• When you are close to the nerve, you will see the color of the posterior ciliary vessels through the connective tissue
• If you become lost, palpate the orbit again and start over. Remind yourself not to dissect past the nerve. There will be times when you unknowingly go past the nerve and see the medial rectus. Palpation will help you avoid this
You will learn to use the visible or palpable landmarks to navigate through the orbital tissues.
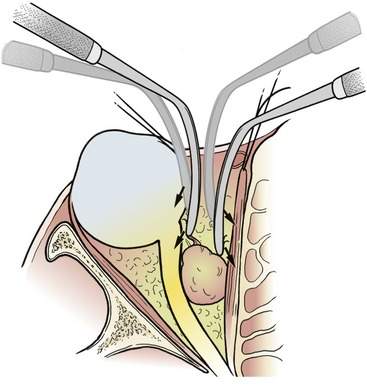
As we discussed in Chapter 1, you cannot underestimate the help that an experienced assistant can give you, especially in a deep orbitotomy. The anticipation and facilitation of the assistant provide you with a third and fourth hand. This is necessary because it is not possible for the surgeon alone to retract the deep wound open and perform a dissection or biopsy. If you are dissecting out an orbital mass:
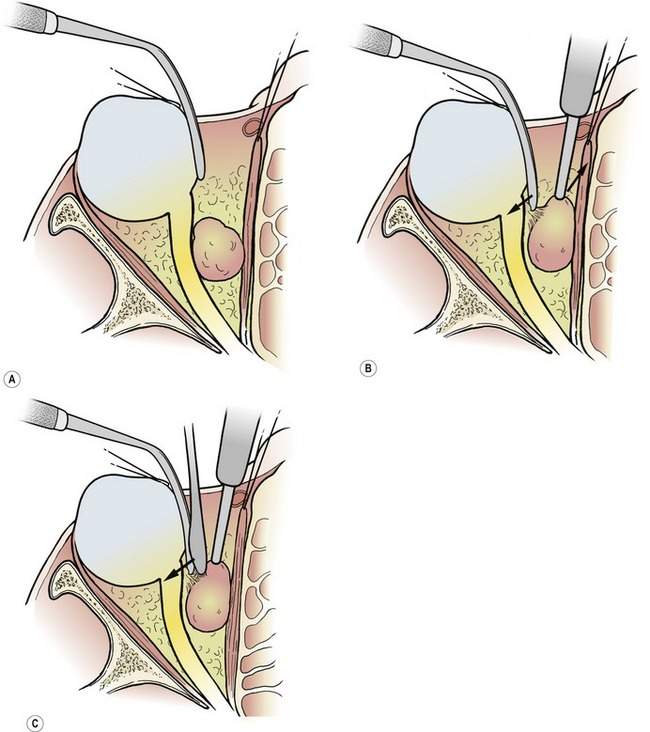
• The assistant should use a retractor to pull the fat away from the mass as you pull the mass away from the fat with your nondominant hand. You can use a forceps, a cotton-tipped applicator, a suction tube, or a retractor
• You can then use your dominant hand to bluntly or sharply separate any bands of tissue on stretch between the fat and the tumor. You can use a pair of scissors or a Freer elevator
This is a basic and important technique for you to know and use for all surgical dissections. If you don’t understand this, ask an experienced surgical colleague to explain it to you. You must understand this technique to function effectively as a surgeon (Figure 15-6). When I am having trouble with an orbital dissection, I remind myself of this basic concept. Probably the tissues are not being pulled apart by either me or my assistant.