18 The deep inferior epigastric artery perforator (DIEAP) flap
Synopsis
 The deep inferior epigastric artery perforator (DIEAP) flap provides a large volume of soft, malleable tissue that resembles the natural consistency of the breast.
The deep inferior epigastric artery perforator (DIEAP) flap provides a large volume of soft, malleable tissue that resembles the natural consistency of the breast.
 DIEAP flap dissection is comparable to conventional myocutaneous free flap surgery, once the initial learning curve is overcome.
DIEAP flap dissection is comparable to conventional myocutaneous free flap surgery, once the initial learning curve is overcome.
 The main advantage of the DIEAP flap is the preservation of full rectus abdominis muscle function translating into less donor site morbidity
The main advantage of the DIEAP flap is the preservation of full rectus abdominis muscle function translating into less donor site morbidity
 In experienced hands, the DIEAP flap loss rate is less than 1%.
In experienced hands, the DIEAP flap loss rate is less than 1%.
 The DIEAP flap is the perforator flap of choice for autologous breast reconstruction.
The DIEAP flap is the perforator flap of choice for autologous breast reconstruction.
Introduction
The deep inferior epigastric artery perforator (DIEAP) flap arose as a refinement of the conventional myocutaneous lower abdominal flap. The myocutaneous perforators of the inferior epigastric vessels were described1 soon after the first transverse rectus abdominis myocutaneous (TRAM) flap was performed for breast reconstruction by Holmström and Robbins.2,3 In the mid-1980s, following Taylor’s landmark work on the vascular territory of the deep inferior epigastric artery, it became apparent that the lower abdominal flap could be perfused solely by a large periumbilical perforating vessel. That assumption was confirmed in 1989 when Koshima and Soeda4 published two cases of “inferior epigastric skin flaps without rectus abdominis muscle”.
History
In 1989, Koshima and Soeda4 reported the first clinical application of the inferior epigastric artery perforator flap. They demonstrated that it was possible to harvest the same amount of lower abdominal skin and fat as in the TRAM flap, without sacrificing the rectus abdominis muscle. Koshima et al.5 then reported another 13 cases with free thin para-umbilical perforator based flaps. Later, Pennington et al.6 used an anastomosis between the distal end of the ipsilateral pedicle and the contralateral pedicle to augment the blood supply of a free TRAM flap. Allen and Treece7 reported 22 successful breast reconstructions with the DIEAP free flap and finally, Blondeel8,9 improved the understanding of the flap and popularized its use in autologous breast reconstruction.10–13
Basic science: anatomy
The deep inferior epigastric artery perforator (DIEAP) flap
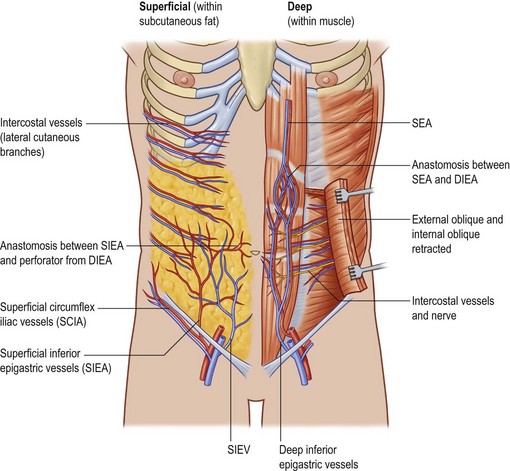
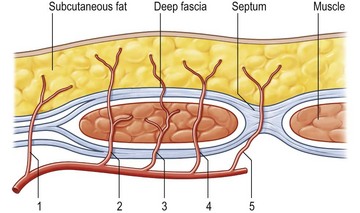
The deep inferior epigastric artery finally divides into numerous branches, which anastomose, above the umbilicus, with the superior epigastric branch of the internal thoracic artery and with the lower intercostal arteries (Figs 18.1, 18.2).
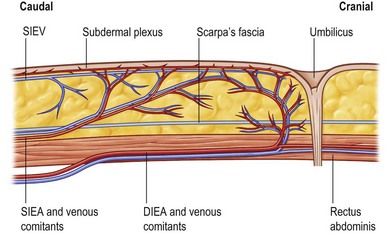
Fig. 18.2 The same anatomical structures as explained in Figure 18.1 but seen in a paramedian sagittal view.
The anatomy of the deep inferior epigastric artery system is very variable.14,15 The average pedicle length is 10.3 cm and the average vessel diameter is 3.6 mm. Normally, the deep inferior epigastric artery divides into two branches, with a dominant lateral branch (54%). However, if the deep inferior epigastric artery does not divide, the vessel has a central course (28%) with multiple small branches to the muscle and centrally located perforators. If the medial branch is dominant (18%), flow appears to be significantly lower than in a central system or in patients with a dominant lateral branch.16
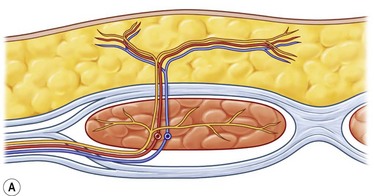
Blondeel et al.16 found between two and eight large (>0.5 mm) perforators on each side of the midline. The majority of these perforators emerged from the anterior rectus fascia in a paramedian rectangular area 2 cm cranial and 6 cm caudal to the umbilicus and between 1 and 6 cm lateral to the umbilicus. Anatomical symmetry was hardly ever encountered. The closer a perforator is to the midline, the better the blood supply to the least vascularized part of the flap across the midline, as one choke vessel less has to be transgressed. However, the lateral perforators are often dominant and easier to dissect because they run more perpendicularly through the muscle. The sensory nerve that runs with these perforating vessels is also often much larger (Fig. 18.3). The medial perforators provide better perfusion of the flap but they have a longer intramuscular course, requiring more elaborate dissection with extensive longitudinal splitting of the muscle. An alternative is to extend the design of the flap to include more tissue from the flank. If one is uncertain as to whether or not enough volume can be transferred, the perforators can be dissected on both sides (Siamese flap).5
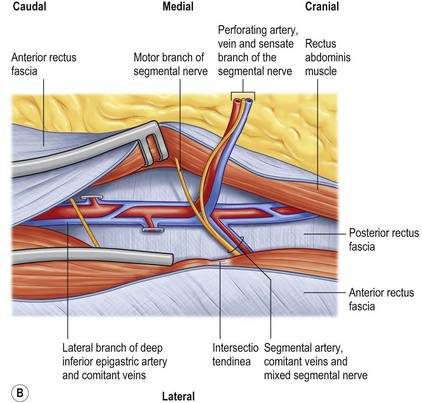
Preference is also given to perforators that pass through the rectus abdominis muscle at the level of the tendinous intersections. At this point, the perforators are frequently large and have few muscular side branches. The distance from the subcutaneous fat to the deep inferior epigastric vessels is also shorter, simplifying this most delicate part of the dissection.17
As a result, the design of a DIEAP flap is made over the most centrally located, dominant perforator, lateral or medial, as long as sufficient abdominal subcutaneous fat tissue is available and the least vascularized part of the flap across the midline can be discarded (Fig. 18.4). At the origin of the perforator, several nerves are encountered (Fig. 18.3). Although there is no constant anatomy, mixed segmental nerves run underneath or through the muscle from laterally and split into a sensate nerve running with the perforator into the flap and a motor nerve crossing on top of the deep inferior epigastric vessels distal to the bifurcation of the perforator, into the medial part of the rectus abdominis muscle.18 One should always expect and anticipate a variety of anatomical differences.
The superficial inferior epigastric artery (SIEA) originates 2–3 cm below the inguinal ligament directly from the common femoral artery (17%) or from a common origin with the superficial circumflex iliac artery (48%). It then passes superiorly and laterally in the femoral triangle lying deep to Scarpa’s fascia and crosses the inguinal ligament at the midpoint between the anterior superior iliac spine and the pubic tubercle. Above the inguinal ligament, the SIEA pierces Scarpa’s fascia and lies in the superficial subcutaneous tissue. During its course the SIEA lies deep to and parallel to the superficial inferior epigastric vein. The vein drains directly into the saphenous bulb.19
The superficial inferior epigastric artery is seen as a direct perforator to the skin while the perforators of the deep system are considered indirect perforators (Fig. 18.5). Of all vessels, it is important to choose the largest, most dominant perforator destined to vascularize the fat and skin, that has few or no side branches to the muscle.
The superficial inferior epigastric vein is the largest vein draining the skin paddle of the DIEAP flap. It is located below the dermal plexus but above Scarpa’s fascia, midway between the anterior superior iliac spine and the pubic symphysis. Harvesting an elliptical skin island transects this vein, redirecting the venous drainage through the smaller perforating veins. Connections between the superficial epigastric vein and the deep inferior epigastric system exist in every patient, but substantial medial branches crossing the midline have been found to be absent in 36% of cases.20,21 In these flaps, venous connections are only present through the subdermal capillary network. This explains why the portion of a flap farthest from the midline may suffer from venous congestion and why the presence of this problem is so variable and unpredictable.
The lymphatic drainage of the DIEAP flap can be divided into a superficial and a deep system. The superficial collectors are located directly underneath the reticular dermis. Deep cuts performed during de-epithelialization may injure this system. The superficial collectors drain to the superficial lymph nodes in the groin. The deep system drains the deep structures of the abdominal wall, i.e., the muscles and fascia and is located in close proximity to the arteries and veins. Careful dissection of the vascular pedicle avoids iatrogenic damage to this lymphatic vasculature. The deep system drains to the inferior epigastric artery and then to the deep iliac nodes.22
Recipient vessels
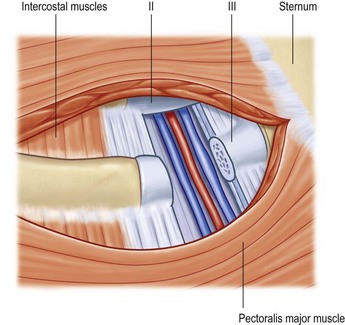
The internal mammary artery and its accompanying veins are the first choice for DIEAP flap breast reconstruction.9,11,23 Its central position on the chest wall facilitates microsurgery and offers the most flexibility during breast shaping. The vessels are easy to dissect and are usually protected from radiotherapy damage. In a number of irradiated vessels perivascular fibrosis can be encountered. Chest wall inflammation, following infected implant removal or extreme capsular fibrosis can sometimes cause severe peri-vascular scarring.
Although the artery is usually of sufficient caliber, the size of the veins is very variable. In general the veins on the left side of the chest wall are smaller than those on the right side. For this reason, we prefer to dissect the vessels at the level of the left third or fourth rib, but at the level of the fourth rib on the right side. A small segment of cartilage can be removed together with some intercostal muscles, both cranially and caudally (Fig. 18.6). This provides sufficient exposure of the vessels and adequate recipient vessel length. One can also limit the dissection and exposure to the removal of only the intercostal muscles. Wider exposure can be obtained by nibbling away the lower border of the superior rib and the upper border of the inferior rib.
Diagnosis/patient presentation
More recently, magnetic resonance angiography has shown promise in the imaging of perforators. In addition to producing accurate and detailed images, there is no radiation exposure,24 unlike CT imaging.
Ultrasound evaluation of perforator vessels
This is performed with a color Doppler, which employs a combination of grayscale and color Doppler imaging. This modality has 100% positive predictive value and few false negatives.25
Grayscale imaging shows the anatomical detail of fixed points, axial vessels and perforating branches. The addition of color Doppler allows identification of blood flow, direction (towards or away from probe), pattern of flow (i.e., venous or arterial) and finally a measure of blood flow velocity.26–29
In addition to preoperative imaging, it is possible to use a unidirectional hand-held pencil probe for identification of superficial vessels in the operating theatre. The perforators identified can be marked on the patient’s skin to allow accurate flap design and aid intraoperative dissection. This is a simple and inexpensive technique, which provides a useful intraoperative adjunct.30 There can, however, be false negative and positive signals as a result of interference from axial vessels or perforators that run parallel to the fascia, before entering their suprafascial course.
CT imaging
Multidetector-row helical CT is a recent innovation that permits rapid delineation of an anatomic area of interest, giving excellent resolution and low artifact rating. It takes less than 10 min to perform and is well tolerated by patients. This has become the modality of choice for the identification of abdominal wall perforators.31–33 The use of magnetic resonance imaging to avoid the high X-ray dose is promising but still needs further sophistication.34
The scanning is performed in conjunction with intravenous contrast medium and allows evaluation of the donor and recipient vessels. Information collected includes the exact location and intramuscular course of vessels from their origin, the caliber of the perforators and also identifies the dominant vessel. Delineation of the relative dominance of the deep and superficial systems allows the surgeon to consider different options preoperatively. Not only can this modality be used to select suitable patients preoperatively but also operative times are reduced by a mean of 21%, with the obvious associated cost benefits.35
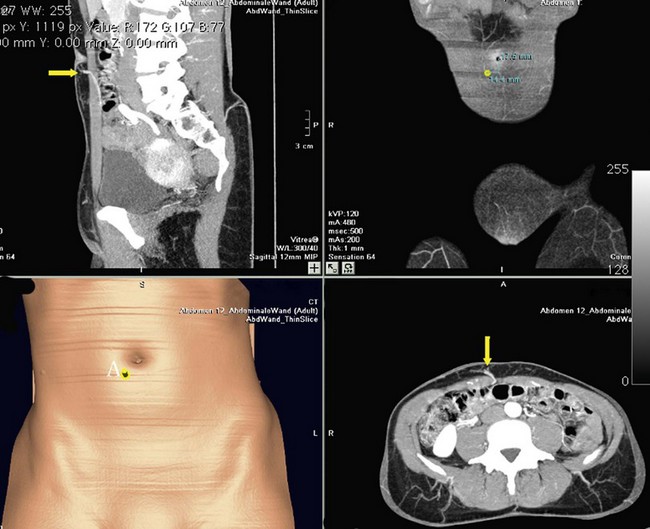
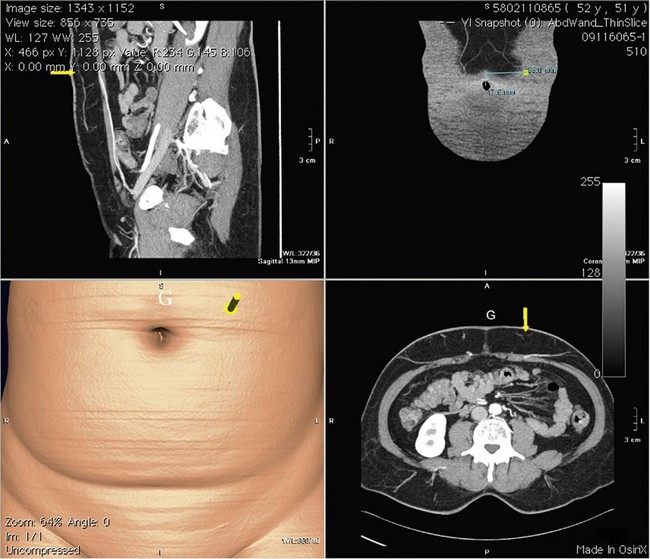
The disadvantages of multidetector-row helical CT lie in the X-ray dosage and use of intravenous contrast media, with the resultant risk of anaphylaxis. The X-ray dose, albeit significant, is less than a conventional liver CT scan and can be combined with staging investigations to reduce the overall exposure. Interpretation of the images can be done before and during surgery by the surgeon him/herself and correlated to intraoperative findings (Figs 18.7, 18.8).