Targeted Muscle Reinnervation and Upper Limb Amputation
Gregory A. Dumanian
INTRODUCTION
Amputation of an upper extremity is a devastating injury from both a functional and psychological standpoint. A major advance in the treatment of upper limb amputees occurred in the past decade with the development of targeted muscle reinnervation (TMR). In this procedure, nerves that had previously controlled the amputated limb are manipulated and transferred to provide signals for a multifunctional myoelectric prosthesis. These procedures have been shown to dramatically improve function after limb loss, and reconstructive plastic surgeons are ideally suited to perform the delicate nerve handling and soft tissue rearrangements required for TMR. This chapter will provide the basis for understanding prosthetic limb control and the level-by-level management of patients with upper limb amputation.
Prosthetic Devices for Upper Limb Amputees
A prosthesis is only as good as the control signals that it receives from the user. Body-powered prostheses utilize shoulder motions for movement. This is problematic, because muscles that are designed for strong movements, such as the latissimus dorsi and the serratus anterior, are required to sensitively activate cables and switches to move the prosthesis in space. Additionally, only one function of the prosthesis can be actuated at a time. Prosthetic hand, wrist, and elbow motions must be performed sequentially, and this slows use of the device to unacceptable levels. It is not surprising that many amputees functionally abandon their prostheses, wearing them only for cosmetic purposes.1
Standard myoelectric prostheses, in comparison, utilize electromyographic (EMG) signals from intact muscles on the limb or shoulder to activate and control prosthetic motion. The muscle activity creates an electric signal that is picked up by sensors and activates motors of the terminal device. For standard myoelectric prostheses, the ease of use depends on the muscles that remain on the amputated limb. For example, in a transradial amputee, native wrist and finger flexors and extensors control the opening and closing of the terminal device, providing a natural pairing of EMG signals and prosthetic movement. For more proximal amputations such as at the transhumeral level, clumsy biceps and triceps muscles are required to open and close the “hand” of the terminal device. The same muscles must also control flexion and extension of the prosthetic elbow. The user must somehow command the device to change from movement of the elbow to movement of the hand, either by muscle co-contraction or by touching a switch. Because this is not intuitive and the hand and elbow movements cannot be performed simultaneously, the device appears jerky and is slow to use. Myoelectric prostheses are heavier, more fragile, and require more daily maintenance than body-powered devices. However, as unilateral upper extremity amputees tend to use the prosthetic limb only as a helper hand, the improved cosmetic appearance of myoelectric devices can make up for their drawbacks and are often preferred.
TARGETED MUSCLE REINNERVATION
In 1995, Kuiken and Childress demonstrated in an animal study a new strategy now called targeted muscle reinnervation for the control of myoelectric prostheses. Rather than using the “wrong” signals from nearby and functionless muscles, the amputated nerve was placed near a denervated muscle in rats. After successful neurotization, the muscle served to amplify the signal of the amputated nerve, and the EMG signal could be detected transcutaneously.2 The downside of this approach is that the amputee requires a surgical procedure and that the intact EMG signal from that muscle is lost for a time until neurotization occurs. The latter tends not to be a problem, given that the muscles “sacrificed” for TMR are not contributing to the function of the limb because the limb is absent. Kuiken and Dumanian reported this procedure in a human shoulder disarticulation patient in 20043 and in transhumeral amputees in 2008.4 The TMR surgical procedure was recently reviewed,5 and this demonstrated improved outcomes in comparison to standard prostheses, with marked improvement (up to 271%, average 198%) in manual dexterity tasks6 and a statistically significant improvement in Assessment of Motor and Process Skills testing (a measure of performance of activities of daily living) when conventionally controlled prostheses were compared with TMR-controlled prostheses. The remarkably smooth coordination of complex tasks has been illustrated in numerous videos.5,7,8 Complications of the procedure have included occasional cellulitis and seroma formation deep to the undermined skin flaps, as well as a transient increase in phantom limb pain.5 Rehabilitation involves socket fitting and optimizing electrode fitting by a skilled prosthetist. Minimal occupational therapy is required because control of the device is more intuitive than with conventionally controlled prostheses.9
Technique
TMR is a technique that transfers the median, radial, ulnar, and/or musculocutaneous nerves in an amputated arm to the small motor nerves of nonfunctional residual limb muscles. These reinnervated target muscles then serve as biological amplifiers of the nerve signals to provide additional EMG control signals and improve control of motorized prostheses. A surgical training video demonstrating key steps in the procedure is available at http://www.ric.org/research/centers/cbm/index.aspx and http://drdumanian.com. Unique features to the procedure that vary based on the level of amputation and individual anatomy are discussed below.
Shoulder Disarticulation Level. TMR at the shoulder level should not be performed unless the surgical team has significant knowledge of upper chest and axillary anatomy.10 Indications for TMR are poor prosthetic function with a standard prosthetic device despite adequate rehabilitation. Patient evaluation begins with a thorough history and physical examination. Patients with brachial plexus injuries are not candidates for the procedure, because the musculocutaneous, median, radial, and ulnar nerves are unable to generate action potentials under direct cortical control. The surgeon
must confirm the presence of Tinel signs for these nerves at the end of the residual limb, indicating that the end neuromas are located close to the end of the residual limb. A history of limb avulsion should alert the team that the nerve endings may be proximally located and not able to reach their targets for transfer. Voluntary contractions of the pectoralis, serratus, and latissimus muscles must also be verified by physical examination both to establish that the entire brachial plexus was not avulsed from the spinal cord and to verify with the patient that these muscle contractions will temporarily be lost after the nerve transfer procedure. Relative contraindications to surgery include a lack of muscle targets, a lack of distally located nerve endings, poor local soft tissues, and the inability to tolerate a 4- to 5-hour surgical procedure.
must confirm the presence of Tinel signs for these nerves at the end of the residual limb, indicating that the end neuromas are located close to the end of the residual limb. A history of limb avulsion should alert the team that the nerve endings may be proximally located and not able to reach their targets for transfer. Voluntary contractions of the pectoralis, serratus, and latissimus muscles must also be verified by physical examination both to establish that the entire brachial plexus was not avulsed from the spinal cord and to verify with the patient that these muscle contractions will temporarily be lost after the nerve transfer procedure. Relative contraindications to surgery include a lack of muscle targets, a lack of distally located nerve endings, poor local soft tissues, and the inability to tolerate a 4- to 5-hour surgical procedure.
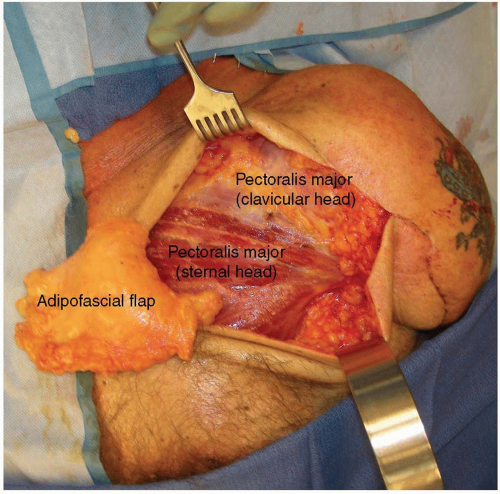
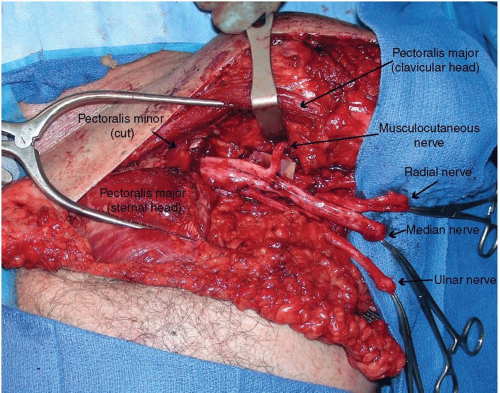
Prior to the skin incision, a dilute epinephrine solution (1:200,000) is injected into the subcutaneous tissues to aid in dissection. An infraclavicular approach to the plexus and proximal nerve branches is performed by incising the skin two fingerbreadths below the clavicle and carefully opening the interspace between the sternal and clavicular heads of the pectoralis major. Thin skin flaps are raised and the subcutaneous chest fat is thinned for improved EMG signal detection over an area of approximately 100 cm2 from the medial chest to the anterior axillary line and from the clavicle inferiorly toward the nipple. An adipofascial flap is then elevated (Figure 90.1) for later placement between the pectoral heads to reduce aberrant reinnervation and to separate the muscle bellies from each other, thereby improving EMG signal differentiation.11 Next, the tissue plane between the clavicular and sternal heads of the pectoralis major is dissected. In this space, the motor nerve to the clavicular head is found, entering the muscle in a vertical direction. Occasionally, a second small motor nerve innervates this muscle. The sternal head generally has a medial branch, a middle branch located medial to the pectoralis minor tendon, a lateral branch that travels through the pectoralis minor muscle, and on occasion a far lateral branch that innervates the lateral most fibers of the muscle. All nerve branches to the pectoralis major must be identified so that the muscle is completely denervated by the end of the procedure. The origin of these motor nerves is irrelevant–only their size and location as they reach the muscle is important as they must be close enough to the mobilized median, radial, ulnar, or musculocutaneous nerves for the transfer. Next, either medial or lateral to the pectoralis minor tendon, the brachial plexus and nerves emanating from it are identified (Figure 90.2). The radial nerve is stimulated to make sure there is no triceps remnant to leave intact (which could be used as an intact signal for prosthetic elbow extension). The identity of the nerves is made by their branching pattern off of
the brachial plexus. Deep to the plexus, the proximal thoracodorsal nerve is identified as another nerve transfer recipient. The donor nerves are cut back proximally as far as possible to reach normal fascicular architecture, while keeping enough length to avoid tension at the coaptation site. Often, 4 to 6 cm of nerve can be trimmed to reach a more normal appearing nerve. The nerve transfer is performed by dividing the small (1 to 1.5 mm) motor nerve innervating the muscle segment and coapting it directly to the large (1 to 1.5 cm) mixed nerve with 6-0 permanent monofilament suture from epineurium to epineurium (Figure 90.3). The site where a motor nerve enters the muscle is termed as “motor point.” A tacking suture into the nearby muscle epimysium helps stabilize and reduce tension on the coaptation site.
the brachial plexus. Deep to the plexus, the proximal thoracodorsal nerve is identified as another nerve transfer recipient. The donor nerves are cut back proximally as far as possible to reach normal fascicular architecture, while keeping enough length to avoid tension at the coaptation site. Often, 4 to 6 cm of nerve can be trimmed to reach a more normal appearing nerve. The nerve transfer is performed by dividing the small (1 to 1.5 mm) motor nerve innervating the muscle segment and coapting it directly to the large (1 to 1.5 cm) mixed nerve with 6-0 permanent monofilament suture from epineurium to epineurium (Figure 90.3). The site where a motor nerve enters the muscle is termed as “motor point.” A tacking suture into the nearby muscle epimysium helps stabilize and reduce tension on the coaptation site.
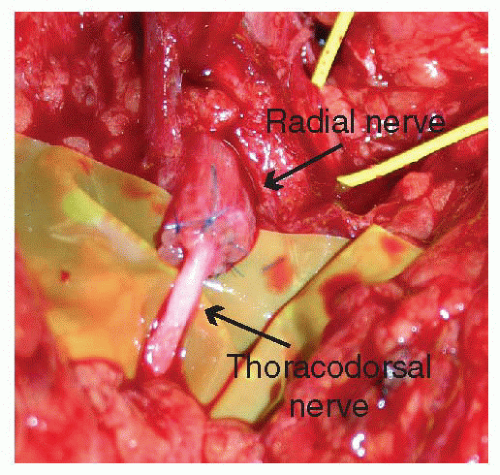 FIGURE 90.3. The epineurium of the radial nerve is coapted to the epineurium of the thoracodorsal target motor nerve using 6-0 permanent monofilament suture. |
Typically, there are four nerves to transfer and there must be four recipients (Figure 90.4). The most common transfers are the musculocutaneous nerve to the motor point of the clavicular head of the pectoralis, the median nerve to the largest motor point innervating the sternal head of the pectoralis, and the radial nerve to the thoracodorsal. In certain cases, the radial nerve is coapted to the long thoracic nerve, or to a nerve innervating the lateral, inferior aspect of the pectoralis major. Ideally, the coaptation occurs directly to the recipient nerve as it enters the muscle (i.e., to the motor point), to minimize the distance required for nerve regeneration. Given the location of the thoracodorsal nerve, there is usually a slightly greater distance from the coaptation site to the latissimus muscle, and as such there may be a longer time period before reinnervation. When available, the best target motor point for the radial nerve is one of the lateral motor nerves to the pectoralis major because they are closer to the target muscle than either the long thoracic or the thoracodorsal nerves are to the serratus and latissimus, respectively. The recipient for the ulnar nerve is generally the motor point found on the lateral and deep aspect of the pectoralis minor. Alternatives for the ulnar nerve include the long thoracic nerve, or the most lateral motor nerve innervating the pectoralis major. If the pectoralis minor is used, it is mobilized laterally and superficially away from the overlying pectoralis major for better signal detection. The sternal and clavicular heads of the pectoralis are routinely separated to provide two targets. The sternal head can be additionally split based on its neurovascular anatomy to create an additional target (Figure 90.4). These segments should be at least 4 to 5 cm in diameter for adequate signal detection. The adipofascial flap is then divided and placed in between the segments to reduce aberrant reinnervation and to physically separate the muscle bellies from each other (Figure 90.5).
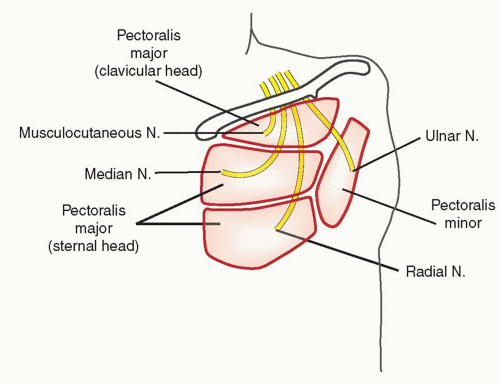 FIGURE 90.4. The typical nerve transfers for a patient with a shoulder disarticulation are illustrated. Ideally, the radial nerve should be coapted to one of the motor points of the pectoralis major, if more than one is identified. Alternatively, the thoracodorsal or long thoracic nerves may be used (not shown), although given the increased distance to their target muscles, the time to reinnervation may be longer.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|