Fig. 24.1
Coronal representation of the development of the frontal and maxillary sinus. The frontal sinus begins to develop at age of 4 and does not reach adulthood until after 12 years old. The newborn (N) has a small maxillary sinus that continues to expand in a lateral inferior direction reaching adult pneumatization after 12 years of age. Note the potential association of the maxillary tooth roots as the maxillary sinus pneumatizes completely. 4, 8 and 12 are represent years of age
The maxillary sinus communicates with the nasal cavity through its ostium. The size of the ostium varies but is generally 1–3 mm in size [4]. The maxillary sinuses have a reliably defined drainage pattern that is based on mucociliary clearance. The maxillary sinus is lined by pseudostratified ciliated columnar epithelium (Fig. 24.2). The cilia beat in a coordinated fashion to transport mucus from the point of its secretion in the sinus toward its natural ostium. The maxillary sinus ostium drains into the ethmoid infundibulum, eventually draining into the middle meatus. Once the secretions have reached the nasal cavity, they are carried into the nasopharynx and then pass into the digestive tract, where the secretions and whatever debris they carry are destroyed.
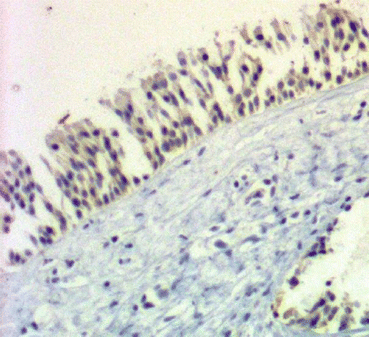

Fig. 24.2
Immunohistochemistry slide demonstrating pseudostratified epithelium from the anterior ethmoid tissue (40× magnification) with human calgranulin (S100A12) staining (innate bacterial peptide)
Lateral Nasal Wall
Understanding the anatomy of the lateral nasal sidewall with its associated anatomical structures, spaces, and sinus ostia is critical for the endoscopic surgeon before surgically approaching the maxillary sinus. Projecting from the lateral nasal sidewall are three conchae or turbinate bones. They are named in ascending sequential order according to their position on the lateral nasal wall. The turbinates in ascending order from inferior to superior are as follows: the inferior turbinate, middle turbinate, superior turbinate, and, if present, there is a fourth turbinate termed the supreme turbinate. Below each turbinate is a meatus or space, whereby its name is derived from the turbinate above. Each meatus receives unique drainage from corresponding paranasal sinuses.
The nasolacrimal duct empties into the inferior meatus, which sits below the inferior turbinate. A small mucosal flap called Hasner’s valve covers the distal opening of the nasolacrimal duct. The nasolacrimal duct is best identified on axial CT cuts and becomes the most anterior limit of dissection when opening the maxillary sinus. The middle meatus is located lateral to the middle turbinate and is the most complex and utmost important to the endoscopic sinus surgeon. The middle meatus receives drainage from the frontal, maxillary, and the anterior ethmoid sinuses. Posteriorly, the superior meatus is below the superior turbinate, which collects drainage from the posterior ethmoid air cells. The drainage continues medially into the sphenoethmoidal recess, which also receives drainage from the sphenoid sinus.
Ostiomeatal Unit
The ostiomeatal unit (OMU) is a complex anatomic area within the middle meatus, which can be defined as the functional designation of the anterior ethmoid complex, thereby acting as the common drainage pathway of the frontal, anterior ethmoid, and maxillary sinuses [5]. The OMU includes the following structures:
Anterior ethmoid cells
Uncinate process
Ethmoid bulla
Ethmoid infundibulum
Hiatus semilunaris
Obstruction of the OMU is commonly the cornerstone seen in the pathophysiology of chronic maxillary sinus disease. Obstruction may be secondary to inflammation or anatomic variations of the OMU such as a paradoxical middle turbinate, concha bullosa, Haller cell, agger nasi cell, or nasal septal deviation. Endoscopic sinus surgery (ESS) specifically addresses the OMU as a functional unit by targeting diseased cells while preserving sinonasal mucosa. This enables the return of normal mucociliary drainage within the OMU. The endoscopic surgeon should carefully evaluate the anatomic variations within the OMU on preoperative imaging to adequately address the underlying disease process. However, there is still a lack of consensus in regard to the role those anatomical variations of the OMU play in the pathogenesis of CRS.
Uncinate Process
The uncinate process is a sickle-shaped bone that appears as a fold on the lateral nasal sidewall that extends from the inferior turbinate to its anterior-superior attachments at the skull base and lamina papyracea. The inferior-posterior most portion of the uncinate has no bony attachment and inserts into the ethmoid complex of the inferior turbinate bone. The middle portion of the uncinate has a bony attachment to the maxillary and lacrimal bones as it extends superiorly. The superior attachment of the uncinate process has a tremendous amount of variation. The location of its attachments has direct consequence on drainage patterns of the frontal sinus and dictates the surgical approach. The uncinate process can also present with pneumatization occluding the infundibulum of the maxillary sinus.
Understanding the variations of the superior insertion of the uncinate process will enable the endoscopic surgeon to protect the frontal sinus drainage pathway when performing an uncinectomy in conjunction with a maxillary antrostomy. Its superior attachment is highly variable and was originally classified with three distinct attachment sites including the lamina papyracea, skull base, and middle turbinate. A more detailed classification has been described classifying the insertion into six different categories [6]. When the uncinate process inserts into the lamina papyracea, the ethmoid infundibulum ends as a blind pouch named the recess terminalis [7]. In this instance, the frontal sinus will drain medially into the middle meatus or the suprabullar recess. However, when the uncinate attaches to either the skull base or the middle turbinate, the frontal recess drains into the middle meatus through the ethmoid infundibulum.
The uncinate process can be atelectatic and intimately opposed to the lamina papyracea seen in conditions such as silent sinus syndrome or chronic maxillary atelectasis, or it can be pushed medially as a result of nasal polyposis (Fig. 24.3). If the laterally rotated uncinate is not recognized, the surgeon may inadvertently enter the orbital cavity as the distance between the uncinate process and the lamina papyracea can be as narrow as 0.1 mm [8]. Likewise, natural congenital dehiscence of the lamina is reported to be as high as 10 % and should be avoided at the time of surgery. Temporally remote trauma can also cause lamina dehiscence, which can alter lateral nasal wall anatomy, causing increased potential for intraoperative injury to orbital contents while performing the uncinectomy. Therefore, careful dissection is required in these instances to prevent lamina penetration and can be assessed intraoperatively with gentle external orbital pressure while visualizing the lamina endoscopically.
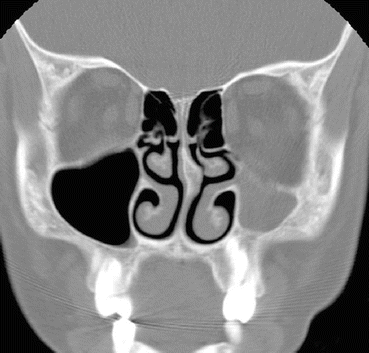

Fig. 24.3
Coronal bone window CT shows an atelectatic left uncinate process that is intimately opposed to the lamina
Infundibulum and Hiatus Semilunaris
The infundibulum is a three-dimensional space that is bounded by the lamina papyracea laterally, the uncinate process medially, and the ethmoid bulla posteriorly. The infundibulum can be likened to a hallway, which collects drainage from the frontal, ethmoid, and the maxillary sinus and subsequently directs the secretions medially to the hiatus semilunaris. The hiatus semilunaris or “exit” is a two-dimensional space that is defined by the free edge of the uncinate and the anterior face of the ethmoid bulla. The hiatus semilunaris can be seen with nasal endoscopy at the most posterior-inferior portion of the uncinate, is difficult to identify on coronal images, and is best seen on sagittal cuts. In contrast, the infundibular space cannot be visualized endoscopically unless the uncinate is removed, which is the first step to surgically access the natural maxillary ostium.
Anatomic Variations
Anatomic variations of sinus anatomy may predispose patients to developing sinus infections. Although the cumulative evidence is unclear in regard to the extent structural changes play in sinonasal pathophysiology, it is apparent that understanding the anatomy and its variations is critical to performing safe endoscopic surgery and improving surgical outcomes. Several key anatomical variations that should be assessed prior to surgery are discussed.
Concha bullosa
Infraorbital recess cells (Haller Cells)
Septal deviation
Concha Bullosa
Zuckerkandl coined the term concha bullosa when pneumatization of the middle turbinate was present. Pneumatization of the middle turbinate can narrow the OMC and can be involved in the pathophysiology of maxillary sinus disease. Large concha bullosa function as large “balloons” in the middle meatus obstructing normal mucociliary drainage. The amount and location of the pneumatization vary, with the most common location being the head of the middle turbinate (Fig. 24.4). This anatomic variation can easily be seen on CT in both coronal and axial views.
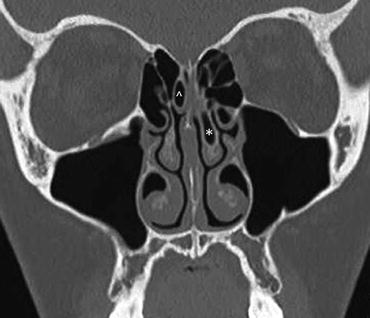

Fig. 24.4
Coronal bone window CT demonstrates pneumatization into the head of the left middle turbinate (*) also called a concha bullosa. There is also a right-sided superior turbinate concha (^)
Infraorbital Ethmoid Cells (Haller Cells)
Infraorbital ethmoid air cells (Haller cells) are seen as pneumatized air cells that grow out of the inferior orbital floor at the roof of the maxillary sinus (Fig. 24.5). Infraorbital ethmoid cells are seen as distinctive air cells separate from the anterior ethmoid bulla. These cells are important to identify as they have the potential to narrow the maxillary sinus drainage, which may predispose the patient to sinusitis [9]. The coronal CT is the best view for identifying these air cells.
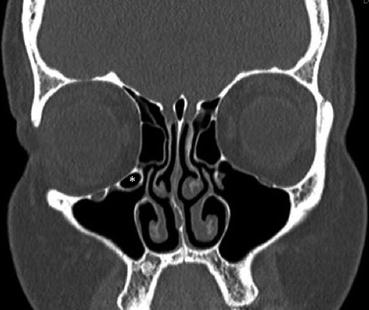

Fig. 24.5
Coronal bone window CT illustrates a right infraorbital ethmoid air cell (Haller cell). Can be visualized as pneumatized air cells of the inferior orbital floor (*)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






