The bearing surface in total hip arthroplasty (THA) is defined by the contacting materials that form the articulation between the reconstructed acetabulum and the replaced femoral head.
ANATOMY
 The native femoroacetabular joint is formed by a nearly frictionless articulation between the cartilage elements of the acetabulum and the femoral head. Upon reconstruction, all remaining cartilage is removed and a new articulation occurs between materials with various mechanical properties.
The native femoroacetabular joint is formed by a nearly frictionless articulation between the cartilage elements of the acetabulum and the femoral head. Upon reconstruction, all remaining cartilage is removed and a new articulation occurs between materials with various mechanical properties.
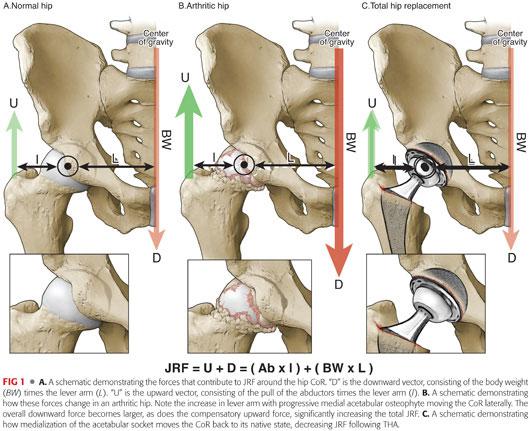
 The relevant anatomy of this articulation involves gravitational and musculotendinous elements that impart forces around the center of rotation (CoR) of the hip: (FIG 1A)
The relevant anatomy of this articulation involves gravitational and musculotendinous elements that impart forces around the center of rotation (CoR) of the hip: (FIG 1A)
The sum of the forces about the hip is zero when rotational and axial stability is present.
The combination of these force vectors results in a joint reactive force (JRF), which is defined as the sum of forces acting on the hip CoR.
The major variables contributing to JRF include the following:
• Body weight (BW)
• Distance from the center of gravity (CoG) to the CoR (the lever arm [L])
It is easiest to consider two main forces acting on the CoR:
• A downward force (D), consisting of the BW times the lever arm for the body weight (L)
• An upward force (U), equal and opposite to D, made up by the pull of the abductor muscles (Ab) times the lever arm for this muscle (l)
• The JRF is the absolute sum of these two forces or essentially force D multiplied by a factor of two.
• This can be represented by the equation:
JRF = (BW × L) + (Ab × l) or
JRF = D + U, and because D must equal U
JRF = 2D
• The greater the BW and the further the CoR is from the CoG (increasing lever arm [L]), the greater D becomes, therefore increasing the JRF.
PATHOGENESIS
 As degenerative arthritis develops in the hip, often an osteophyte forms along the medial wall of the acetabulum, causing lateral migration of the femoral head and the CoR.
As degenerative arthritis develops in the hip, often an osteophyte forms along the medial wall of the acetabulum, causing lateral migration of the femoral head and the CoR.
This creates a vicious cycle whereby the lever arm increases, as does the JRF, causing further buildup of medial osteophyte and further lateralization of the femoral head (FIG 1B).
In addition, the contact area between the acetabulum and the femoral head is reduced, further increasing contact stresses on the articular surface.
 Increasing JRF can result in pain, decreased function, and an accelerated rate of cartilage degradation.
Increasing JRF can result in pain, decreased function, and an accelerated rate of cartilage degradation.
 In addition, patients will often reflexively lean over the affected hip, attempting to move their CoG toward the hip CoR, decreasing the lever arm, JRF, and pain.
In addition, patients will often reflexively lean over the affected hip, attempting to move their CoG toward the hip CoR, decreasing the lever arm, JRF, and pain.
This leads to an altered gait pattern called a Trendelenburg gait.
A Trendelenburg gait pattern also lessens the workload of the abductor by decreasing the amount of force needed to counteract downward force.
Over time, this can lead to significant atrophy of the abductor and persistent dysfunction, even following THA.
 The concept of JRF remains relevant during and following THA, as the JRF following THA equates to the force on the bearing surface.
The concept of JRF remains relevant during and following THA, as the JRF following THA equates to the force on the bearing surface.
The greater the JRF following THA, the greater the forces on the bearing surface and theoretically the higher the rate of wear and osteolysis.
Medialization of the socket during THA has the effect of decreasing the body weight lever arm, thereby decreasing JRF on the bearing surface and decreasing the rate of wear (FIG 1C).
 Following THA, increases in JRF can lead to accelerated production of wear particles, causing several potential problems:
Following THA, increases in JRF can lead to accelerated production of wear particles, causing several potential problems:
Osteolysis
Increased risk of component loosening and failure
Increased risk of component fracture
Increased risk of modular junction issues (eg, taper corrosion)
NATURAL HISTORY
 Because cartilage has little restorative capacity, the natural history of arthritis is typically progressive dysfunction and gradually worsening pain over time.
Because cartilage has little restorative capacity, the natural history of arthritis is typically progressive dysfunction and gradually worsening pain over time.
 Progressive biomechanical and structural changes include the following:
Progressive biomechanical and structural changes include the following:
Cartilage delamination
Osteophyte formation
Lateral migration of the femoral head and hip CoR
Abductor weakness
Ambulatory dysfunction
 Following THA, the bearing surface is subjected to the same principles determining JRF as the cartilage in the native joint.
Following THA, the bearing surface is subjected to the same principles determining JRF as the cartilage in the native joint.
Like any nonbiologic material, the materials used in THA break down over time via a process generically termed wear.
 Regardless of bearing surface materials, over time, wear particles are released into the local tissues following THA and can cause:
Regardless of bearing surface materials, over time, wear particles are released into the local tissues following THA and can cause:
Osteolysis
Synovitis and local tissue irritation
Metal ion-related pathology
Systemic side effects
PATIENT HISTORY AND PHYSICAL FINDINGS
 Frequently, osteolysis is asymptomatic and seen either incidentally on plain radiographs or noticed on routine, scheduled follow-up films.
Frequently, osteolysis is asymptomatic and seen either incidentally on plain radiographs or noticed on routine, scheduled follow-up films.
 If osteolysis is severe, it can cause loosening of the prosthesis, which can manifest as:
If osteolysis is severe, it can cause loosening of the prosthesis, which can manifest as:
Pain in the groin or thigh
• Thigh pain typically correlates with stem loosening.
• Groin pain typically correlates with cup loosening or synovitis.
• Pain worsens over time.
“Start-up” pain
• Pain that is significant at the beginning of ambulation but recedes with continued ambulation
• Usually secondary to initial settling of a loose prosthesis
 Wear particles can produce synovitis in the hip that may manifest as:
Wear particles can produce synovitis in the hip that may manifest as:
Groin pain
Weakness
Joint swelling
 Physical examination findings consistent with advanced osteolysis and/or loosening include the following:
Physical examination findings consistent with advanced osteolysis and/or loosening include the following:
Pain with ambulation
Pain with active hip flexion, particularly active straight-leg raising
Swelling
Tenderness to palpation
IMAGING AND OTHER DIAGNOSTIC STUDIES
 As bearing surface wear and osteolysis are often asymptomatic, it is important to take serial plain radiographs at routine intervals following THA.
As bearing surface wear and osteolysis are often asymptomatic, it is important to take serial plain radiographs at routine intervals following THA.
With the use of conventional highly cross-linked polyethylene (PE), routine follow-up films should be obtained 5 years postoperatively.17,27
• Subsequent films should be taken every 2 to 3 years thereafter.
• If an osteolytic lesion is noted, serial radiographs should be obtained at least every year and occasionally every 6 months, depending on the size and extent of the lesion.
The sensitivity of plain radiographs is quite low, particularly with respect to detecting smaller lesions.
 Computed tomography (CT) can be very useful for the detection and monitoring of osteolytic lesions about the pelvis15 (FIG 2).
Computed tomography (CT) can be very useful for the detection and monitoring of osteolytic lesions about the pelvis15 (FIG 2).
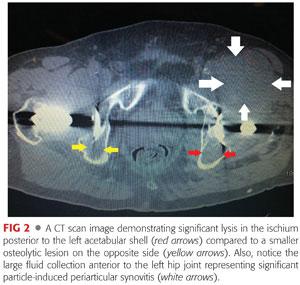
CT is often used to:
• Detect smaller lytic lesions
• Determine component position
• Look for evidence of soft tissue masses or fluid collections about the hip
 Magnetic resonance imaging (MRI) is of limited value following THA due to the significant artifact produced by the metal components.
Magnetic resonance imaging (MRI) is of limited value following THA due to the significant artifact produced by the metal components.
Newer MRI sequences that reduce the amount of artifact (metal artifact reduction [MAR] MRI) can be useful, particularly when evaluating for pseudotumor in the soft tissues surrounding a THA. Findings can include the following:
• Solid or cystic masses
• Muscle wasting
• Soft tissue destruction
 Bone scans can be useful means for the detection or exclusion of loosening but should not be used as the primary diagnostic tool.
Bone scans can be useful means for the detection or exclusion of loosening but should not be used as the primary diagnostic tool.
Bone scans often do not differentiate between lysis and loosening.
The specificity and sensitivity of bone scans is lacking.
 Laboratory studies are often used to distinguish between infection, wear, and aseptic loosening and should include the following:
Laboratory studies are often used to distinguish between infection, wear, and aseptic loosening and should include the following:
Complete blood count (CBC) with differential, which is often normal but can be elevated with significant infection
Erythrocyte sedimentation rate (ESR), which is often normal in cases of aseptic loosening but elevated with infection
C-reactive protein (CRP), which is also often normal in cases of aseptic loosening but elevated with infection
Serum cobalt and chromium levels (in metal on metal articulations or to evaluate for corrosion at modular junctions)
DIFFERENTIAL DIAGNOSIS
 The major materials used for THA bearing surfaces include the following:
The major materials used for THA bearing surfaces include the following:
PE
Cobalt-chromium (CoCr)
Ceramic
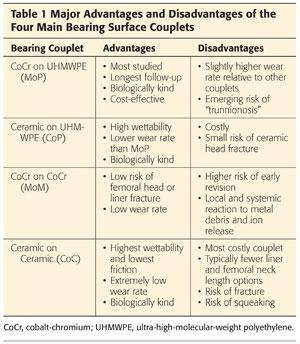
 These materials are combined to form four major bearing surface couplets:
These materials are combined to form four major bearing surface couplets:
CoCr femoral heads articulating with PE acetabular liners (MoP)
Ceramic femoral heads articulating with PE acetabular liners (CoP)
CoCr femoral heads articulating with CoCr acetabular liners (MoM)
Ceramic femoral heads articulating with ceramic acetabular liners (CoC)
 Each of these bearing couplets has distinct advantages and disadvantages (Table 1).
Each of these bearing couplets has distinct advantages and disadvantages (Table 1).
NONOPERATIVE MANAGEMENT
 Management of asymptomatic, stable osteolysis and wear consists of serial radiographs and laboratory studies, regular follow-up, activity modification, and limited use of anti-inflammatory medications.
Management of asymptomatic, stable osteolysis and wear consists of serial radiographs and laboratory studies, regular follow-up, activity modification, and limited use of anti-inflammatory medications.
If asymptomatic osteolysis becomes progressive, surgery may be indicated to prevent further bone destruction and potentially more complicated revision surgery.
 Symptomatic osteolysis is typically severe and often requires operative management.
Symptomatic osteolysis is typically severe and often requires operative management.
Exceptions to this rule include the following:
• Older patients with prohibitive medical comorbidities
• Minimal or nonambulators
• Extreme osteolysis that makes reconstruction impossible
SURGICAL MANAGEMENT
 The goal of THA is to restore as closely as possible the natural biomechanics of the hip joint, allowing for a return to normal gait and improved function, pain relief, and quality of life.
The goal of THA is to restore as closely as possible the natural biomechanics of the hip joint, allowing for a return to normal gait and improved function, pain relief, and quality of life.
Restoration of the hip CoR, native femoral offset, and limb length is critical.
 Appropriate choice of bearing surface options is an important component to the overall success of THA.
Appropriate choice of bearing surface options is an important component to the overall success of THA.
Preoperative Planning
 Choice of bearing surface is usually determined preoperatively and depends on the following:
Choice of bearing surface is usually determined preoperatively and depends on the following:
Patient characteristics that contribute to the expected overall lifetime wear of the prosthesis, including the following:
• Age
• Body weight
• Life expectancy
• Activity level
• Generally speaking, the younger and more active the patient, the more consideration that should be given to alternative bearing surfaces (options other than MoP).
Technical considerations
• Larger femoral heads offer enhanced stability but typically higher wear rates.
• Larger femoral heads also impart greater shear force to the modular trunnion and potentially increase the risk of fretting corrosion at the modular interface.
• Alternative bearing surfaces may not be available with as many options as MoP.
• Some neck length options necessitate the need for skirts, decreasing the head–neck ratio and range of motion to impingement.
Cost
• Ceramic components tend to be more expensive, whereas metal femoral heads and PE liners are usually the least expensive.
• Cost will likely become increasingly important as health care systems attempt to address the growing need for THA with limited available funds and begin to consider demand matching when choosing devices.
Tribology
 Tribology is defined as the study of design, friction, wear, and lubrication of interacting surfaces in relative motion, which is critical for the understanding of bearing couplets in THA.
Tribology is defined as the study of design, friction, wear, and lubrication of interacting surfaces in relative motion, which is critical for the understanding of bearing couplets in THA.
 Conceptually, friction can be thought of as the force resisting motion between two surfaces.
Conceptually, friction can be thought of as the force resisting motion between two surfaces.
The coefficient of friction (CoF) is a value used to compare friction of different surface couplets (Table 2).
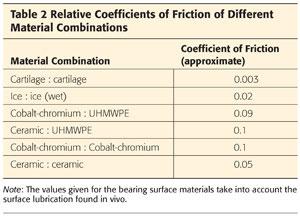
• It is a ratio of the force needed to produce movement, divided by the normal force between the surfaces.
• The lower the CoF, the less friction there is between two bodies.
In THA, friction results in the delamination of material from the surface of the bearings, the major cause of wear, and subsequent osteolysis.
The CoF in THA bearings is influenced by several factors, including the following:
• Contact area
• Clearance
• Surface roughness
• Lubrication
 Contact area and clearance
Contact area and clearance
Contact area is inversely proportional to clearance.
In general, lower clearance and higher contact area are preferred.
• Higher contact area leads to lower contact stress.
• Lower contact stress leads to lower rates of wear.
• Lower clearance leads to more favorable lubrication dynamics.
However, clearance below a certain point can lead to extremely high friction.
• No potential space for fluid leads to complete loss of lubrication.
• Direct contact between the surfaces (equatorial contact)
 Surface roughness
Surface roughness
Can be thought of as the smoothness of the bearing surface
At a microscopic level, the surface of any material is not completely uniform, with pits and projections into and out from the intended diameter.
The more uniform the diameter (ie, fewer pits and projections), the less the surface roughness and the less friction and wear that occurs.
Surface roughness can change over time.
• As opposed to hard-on-soft bearing couplets (ie, MoP and CoP), hard-on-hard bearing couplets (ie, MoM and CoC) can have an initial run-in phase, during which surface roughness is reduced.
• The harder the femoral head, the less susceptible it will be to scratching from third bodies (ie, bone debris within the articulation), which can negatively impact surface roughness after implantation.
 Lubrication occurs when a film of fluid is interposed between two surfaces.
Lubrication occurs when a film of fluid is interposed between two surfaces.
Fluid film lubrication in THA can be full or mixed.
• Mixed-fluid film lubrication results in variable degrees of surface contact, whereas full-fluid film lubrication completely separates the two surfaces and reduces wear.
• To a certain degree, fluid film lubrication permits small variations in surface roughness by slightly separating the bearing surfaces.
Lubrication is influenced by several factors:
• Fluid viscosity, with higher viscosity leading to a more favorable lubrication scheme
• Clearance between the two articulating surfaces
• Surface material and wettability
• Wettability refers to the ability of fluid to interact with another material and improves lubrication by allowing a more uniform fluid layer.
• CoC couplets tend toward full lubrication, whereas MoM, CoP, and MoP couplets tend toward mixed lubrication.10
TECHNIQUES
 Bearing Surface Options
Bearing Surface Options
Polyethylene
PE has been in use for THA since the early 1960s.
Traditional PE is made of ultra-high-molecular-weight polyethylene (UHMWPE).
UHMWPE is composed of ethylene polymer chains.
Contains two major structural regions:
• Crystallites are regions of folded chains that confer the mechanical properties to the material.
• Amorphous regions constitute the remainder of the material and do not contribute significantly to the mechanical properties.
PE technology has evolved; today, most UHMWPE used for THA uses cross-linking technology to improve wear characteristics.
Cross-linking refers to a process by which adjacent ethylene polymers are covalently bonded together (TECH FIG 1A).
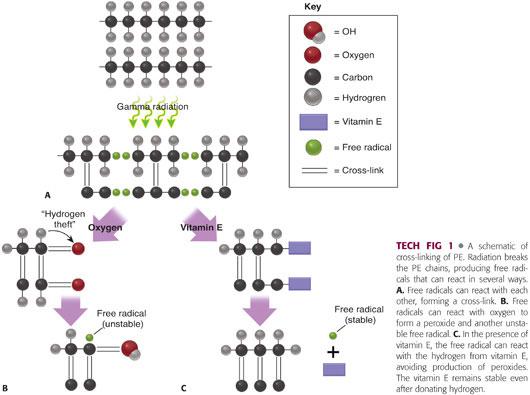
• Cross-linking is usually achieved using either gamma or electron-beam irradiation.
• Radiation causes the formation of free radicals that have the potential to join together in a covalent bond between polymers.
• Cross-linking occurs predominantly in the amorphous regions.
• The amount of cross-linking is proportional to the dose of radiation.
The advantages of cross-linking include the following:
• Decreased wear
• Decreased risk of osteolysis
• Improved material longevity
The disadvantages of cross-linking include the following:
• Alteration of mechanical properties, specifically decreased fatigue and fracture resistance, especially when subject to impingement following component malposition in THA11
• Any residual free radicals can lead to oxidation of the PE and early failure.
Oxidation is one of the main causes of PE failure.
Occurs when free radicals react with oxygen18 (TECH FIG 1B)
• Forms peroxy free radicals which steal hydrogen from other ethylene chains, forming unstable hydroperoxides
• Hydroperoxides then react with ethylene polymers and degrade into products (ketones, acids, esters) associated with diminution of PE mechanical properties.
• The hydrogen theft creates another free radical to start this process over again, leading to a vicious cycle of PE deterioration.
Any process that results in the creation of free radicals can lead to oxidative instability and the risk of PE failure.
• Cross-linking
• Gamma sterilization
Oxidation can occur during or after manufacturing, including in vivo following component implantation.
Manufacturers take advantage of several technologies to reduce the risk of oxidation while improving the wear characteristics of PE:
Sterilization technique
• Conventional PE historically was sterilized using gamma irradiation in air (high oxygen content) which led to high levels of early oxidation and failure.
• Newer generation PE is often sterilized with gas to reduce free radical production.
• Sterilization also now takes place in an inert environment (typically nitrogen) to eliminate early oxidation.
Thermal treatment
• Heating of PE following irradiation converts the crystalline regions to amorphous matter, allowing free radicals to migrate and bond with each other.
• Annealing is performed by heating the PE to just below its 137° C melting point, which allows the PE to retain its mechanical properties, but results in a lower degree of free radical reduction.
• Melting heats the PE to above the melting point and permits the conversion of all crystalline matter to amorphous matter, resulting in a higher degree of free radical elimination, but some loss of crystallization and ultimate mechanical strength.
Vitamin E infusion
• Vitamin E is a natural scavenger of free radicals and is present throughout the body to protect tissues from oxidative degradation.
• Vitamin E can be added to PE in several different manners, leading to either a surface concentration of vitamin E or an even distribution of vitamin E throughout the PE structure.
• “Diffusion” refers to the postmanufacturing addition of vitamin E, whereas “blending” refers to the addition of vitamin E to PE resin during the manufacturing process.
• In UHMWPE, vitamin E lends hydrogen to peroxy free radicals eliminating the free radical, avoiding oxidation, and halting the cycle of free radical production (TECH FIG 1C).
Cobalt-Chromium
CoCr is a group of alloys using different proportions of cobalt and chromium, in addition to other metals typically including nickel and molybdenum.
Cobalt provides the mechanical strength and hardness to the material, whereas the chromium is soluble in the cobalt and confers resistance to corrosion.
Different concentrations of metals results in varying degrees of strength and resistance to corrosion.
CoCr has been used to manufacture both femoral heads and acetabular components and can serve as the acetabular bearing surface.
CoCr is currently the material most commonly used for femoral heads.
• It has better hardness compared to titanium and therefore is more resistant to in vivo conformational changes and third body damage that can increase wear.
The use of CoCr as an acetabular liner has rapidly decreased recently due concerns about local and systemic reactions to metal wear products.
Ceramics
Bearing surface ceramics can be manufactured using both aluminum and zirconium oxide.
Ceramics are made from crystalline powders that are sintered into their general shape.
The crystallites within the structure are referred to as grains.
Grain size is inversely proportional to material strength.
• Higher grain size results in lower strength and decreased fracture resistance.
• Reduced grain size results in higher strength and enhanced fracture resistance.
Advantages of ceramics include the following:
Increased hardness, allowing for finer material polishing and enhanced scratch resistance
Increased wettability, improving lubrication characteristics
Decreased wear
Disadvantages of ceramics include the following:
Currently, there are fewer neck length and liner options.
Decreased ductility and increased brittleness
Risk of possible fracture and catastrophic failure
Risk of bearing surface noise including squeaking
There are two major types of ceramics used today for THA, both of which are made and distributed by one company (CeramTec, Plochingen, Germany).
Pure alumina oxide (Biolox forte), introduced in 1994
Aluminum oxide matrix (Biolox delta), approved in the United States in 2003
• The most recent generation of ceramic bearing surfaces.
• In addition to aluminum, this material contains yttria-stabilized zirconium and platelet-shaped strontium oxide particles which improve the mechanical properties of the ceramic by decreasing the risk of both fracture initiation and propagation.
PEARLS AND PITFALLS | |
Pearls | Pitfalls |
Choice of bearing surface should be made preoperatively, with proper consideration of intraoperative backup plans. |
|
Proper component orientation is imperative for all bearing surfaces. |
|
Choice of bearing surface is not a one size fits all proposition. |
|
Routine follow-up is essential. |
|
MoM have been noted to demonstrate significantly higher rates of early failure. |
|
Squeaking following CoC THA may be an indication of impending failure. |
|
POSTOPERATIVE CARE
 Postoperative care following THA should follow specific standards, regardless of bearing surface choice.
Postoperative care following THA should follow specific standards, regardless of bearing surface choice.
 Early postoperative visits should focus on the following:
Early postoperative visits should focus on the following:
Wound healing
Early radiographic stability of components
• Lack of subsidence
• Appropriate component position
Progression of activities, relief of pain, and improvement in quality of life
 Later postoperative visits should concentrate on detecting signs of component failure such as the following:
Later postoperative visits should concentrate on detecting signs of component failure such as the following:
Osteolysis
Eccentric PE wear
Instability
Pain
Bearing noise
Biologic reactions to metal debris
OUTCOMES
 CoCr and CoP
CoCr and CoP
Most commonly used bearing couplet
Excellent long-term outcomes for both cross-linked and traditional PE acetabular liners
Larger femoral heads (≥36 mm) in this cohort impart substantially improved stability but at a cost of higher volumetric wear rates than smaller head sizes.20
Cross-linked PE liners and ceramic femoral heads are more costly than their conventional counterparts, but midterm clinical data have demonstrated that they have distinct advantages.
Advantages of cross-linked PE over conventional PE liners are that7,9,14,29:
• Wear rate is approximately 8- to 10-fold lower.
• Lower incidence of osteolysis
• Fewer revisions due to osteolysis
Advantages of ceramic femoral heads versus CoCr femoral heads may include the following:
• Lower wear rates22,28
• Elimination of chrome and cobalt leads to a lower risk of femoral neck taper corrosion (trunnionosis).19
 CoC
CoC
Exceedingly low wear rates
Early generation ceramics demonstrate long-term survival (>20 years) of greater than 84%, with osteolysis and loosening accounting for the majority of revisions.25
Newer generation ceramics show excellent midterm survival rates, demonstrating greater than 98% survival at 10 years.5,32
Reasons for aseptic THA failure include the following:
• Osteolysis
• Loosening
• Squeaking
• Component fracture
 MoM
MoM
Reported advantages include the following:
• Decreased wear
• Low risk of fracture
• Low dislocation rates (secondary to increased femoral head diameter)
Use has dwindled in recent years out of concern for early failure and adverse local tissue reaction (ALTR) related to metal wear particles.
Demonstrates significantly lower survival rates compared to other bearing couplets.23,30
Most common reasons for failure include the following:
• Early osteolysis
• Early loosening
• ALTR
Should be used with caution due to reports of higher early failure compared to other bearing couplets. It is not yet clear whether certain subsets of patients are more susceptible than others to early failure when MoM constructs are used.4
COMPLICATIONS
 Osteolysis is the progressive deterioration of periprosthetic bone following joint arthroplasty.
Osteolysis is the progressive deterioration of periprosthetic bone following joint arthroplasty.
It is typically caused by the biologic response to small wear particles.8
• Can occur with any bearing couplet
• The degree of osteolysis depends on the biologic activity of wear particles.
• Particle material is an important characteristic in their biologic activity, with chrome and cobalt particles being particularly active.
• Size is also an important determinant of biologic activity and it is commonly thought that particles between 0.2 and 7 μm in diameter create the highest degree of biologic activity.
Degradation of bone is a side effect of the body’s attempt to eliminate small wear particles.
Osteolysis is a primary cause of aseptic loosening and periprosthetic fracture (FIG 3).
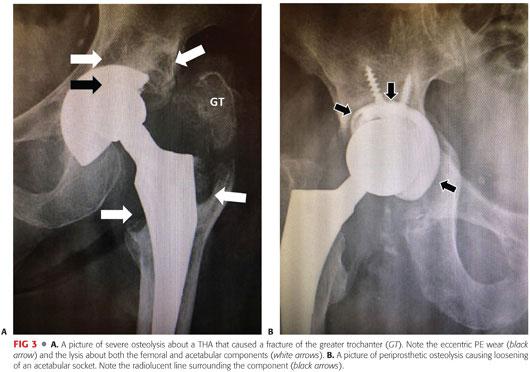
It is often asymptomatic until complications occur (ie, painful loosening).
The development of osteolysis is closely associated with wear debris particles and their effect on cell differentiation, cell proliferation, and cytokine regulation.12,24
• Increased proliferation of osteoclasts
• Decreased proliferation of osteoblasts
• Upregulation of interleukin-1(IL-1) and tumor necrosis factor alpha (TNF-α), amongst other proinflammatory cytokines
• Downregulation of procollagen α-1
Increased rates of wear and lysis is associated with the following:
• Poor component position
• Manufacturing techniques
• Increased body weight and activity level21
 ALTR and pseudotumor
ALTR and pseudotumor
Thought to develop from release of local metal debris
Can occur in any type of hip replacement construct, but the risk is dramatically higher in cases involving:
• MoM articulations, with a reported incidence as high as 6.5% in this population33
• Increased modularity (ie, modular neck/stem constructs), particularly when dissimilar metals are mated at the modular junction
Significant local pathology resulting in the following:
• Soft tissue compromise including muscle, tendon, and bone (FIG 4)
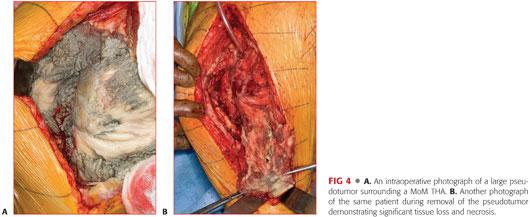
• Mass effect from solid, cystic, or mixed tumors
• Pain
• Osteolysis
• Loosening
Although definite risk factors are poorly understood, certain constructs should be considered hips at risk (FIG 5).

Patients can often present with pain and/or hip dysfunction but can be asymptomatic.
Clinical evaluation of these patients should include the following:
• Thorough history and physical examination, specifically considering:
• Pain
• Ambulatory dysfunction
• Abductor insufficiency
• Soft tissue swelling
• History of instability
• Imaging, including the following:
• Plain x-rays
• CT scan
• MRI using a metal artifact reduction series (MARS), looking for evidence of space-occupying lesion and periarticular tissue destruction (FIG 6)
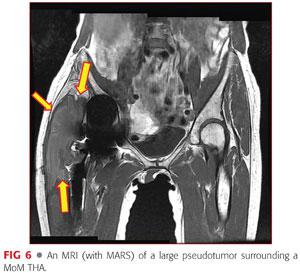
• Lab work should include the following:
• ESR
• CRP
• Serial serum cobalt and chromium levels
• Hip aspiration with cell count, differential, and gram stain/culture
Treatment typically requires revision surgery such as the following:
• Exchange of metal components to either PE or ceramic when possible
• Dèbridement of all necrotic tissue
• The possibility of the need for full revision surgery, including the use of constrained components if necessary secondary to soft tissue compromise
 Bearing surface fracture
Bearing surface fracture
Occurs almost exclusively with ceramic components
Overall incidence of fracture is low.
• Reported to be between 0% and 2.6% in clinical series3,13,26
• More commonly involves the acetabular liner but can also involve the femoral head
• Increased risk with component malposition and resultant impingement
Newer generation ceramics are believed to have a reduced risk of fracture due to the following:
• Smaller grain size
• Stabilizing particles mixed into components to prevent fracture initiation and propagation.
Principles of treatment include the following:
• Immediate joint immobilization and bed rest to prevent fragment dispersal and destruction of well-fixed components
• Urgent revision
• Total synovectomy with removal of ceramic debris
• Profuse joint lavage
Studies suggest that outcomes for revision following fractured ceramic components may be improved with newer ceramics.31
Complete component revision may be necessary as opposed to simple head and liner revision in some cases.2
 Squeaking and bearing surface noise
Squeaking and bearing surface noise
Highest incidence found with CoC constructs.
Risk factors and mechanism are poorly understood, but edge loading seems to be a critical factor.
Overall reported incidence varies between less than 1% and 10%, depending on study methodology (eg, definition of noise).16 Higher rates are noted when patients are specifically asked about noises.
Squeaking is often associated with stripe wear found at the time of revision6 (FIG 7). It is thought to be connected with lubrication that allows surface contact and metal transfer.

Squeaking can be an early indicator of impending liner fracture and should be worked up and followed carefully.1
REFERENCES
1. Abdel MP, Heyse TJ, Elpers ME, et al. Ceramic liner fractures presenting as squeaking after primary total hip arthroplasty. J Bone Joint Surg Am 2014;96(1):27–31.
2. Allain J, Roudot-Thoraval F, Delecrin J, et al. Revision total hip arthroplasty performed after fracture of a ceramic femoral head. A multicenter survivorship study. J Bone Joint Surg Am 2003;85-A(5):825–830.
3. Amanatullah DF, Landa J, Strauss EJ, et al. Comparison of surgical outcomes and implant wear between ceramic-ceramic and ceramic-polyethylene articulations in total hip arthroplasty. J Arthroplasty 2011;26(6 suppl):72–77.
4. Bozic KJ, Browne J, Dangles CJ, et al. Modern metal-on-metal hip implants. J Am Acad Orthop Surg 2012;20(6):402–406.
5. Brandt JM, Gascoyne TC, Guenther LE, et al. Clinical failure analysis of contemporary ceramic-on-ceramic total hip replacements. Proc Inst Mech Eng H 2013;227(8):833–846.
6. Chevillotte C, Trousdale RT, Chen Q, et al. The 2009 Frank Stinchfield Award: “hip squeaking”: a biomechanical study of ceramic-on-ceramic bearing surfaces. Clin Orthop Relat Res 2010;468(2):345–350.
7. Digas G, Kärrholm J, Thanner J, et al. The Otto Aufranc Award. Highly cross-linked polyethylene in total hip arthroplasty: randomized evaluation of penetration rate in cemented and uncemented sockets using radiostereometric analysis. Clin Orthop Relat Res 2004;(429):6–16.
8. Dowd JE, Cha CW, Trakru S, et al. Failure of total hip arthroplasty with a precoated prosthesis. 4- to 11-year results. Clin Orthop Relat Res 1998;(355):123–136.
9. Engh CA, Hopper RH, Huynh C, et al. A prospective, randomized study of cross-linked and non-cross-linked polyethylene for total hip arthroplasty at 10-year follow-up. J Arthroplasty 2012;27(8 suppl):2–7.
10. Flanagan S, Jones E, Birkinshaw C. In vitro friction and lubrication of large bearing hip prostheses. Proc Inst Mech Eng H 2010;224(7):853–864.
11. Gencur SJ, Rimnac CM, Kurtz SM. Fatigue crack propagation of virgin and highly cross-linked, thermally treated ultra-high molecular weight polyethylene. Biomaterials 2006;27(8):1550–1557.
12. Goodman SB, Huie P, Song Y, et al. Cellular profile and cytokine production at prosthetic interfaces. Study of tissues retrieved from revised hip and knee replacements. J Bone Joint Surg Br 1998;80:531–539.
13. Hannouche D, Nich C, Bizot P, et al. Fractures of ceramic bearings: history and present status. Clin Orthop Relat Res 2003;(417):19–26.
14. Harsha AP, Joyce TJ. Comparative wear tests of ultra-high molecular weight polyethylene and cross-linked polyethylene. Proc Inst Mech Eng H 2010;227(5):600–608.
15. Howie DW, Neale SD, Martin W, et al. Progression of periacetabular osteolytic lesions. J Bone Joint Surg Am 2012;94(16):e1171–e1176.
16. Jarrett CA, Ranawat AS, Bruzzone M, et al. The squeaking hip: a phenomenon of ceramic-on-ceramic total hip arthroplasty. J Bone Joint Surg Am 2009;91(6):1344–1349.
17. Kitamura N, Sychterz-Terefenko CJ, Engh CA. The temporal progression of pelvic osteolysis after uncemented total hip arthroplasty. J Arthroplasty 2006;21(6):791–795.
18. Kurtz SM. The UHMWPE Biomaterials Handbook: Ultra High Molecular Weight Polyethylene in Total Joint Replacement and Medical Devices, ed 2. Burlington, MA: Academic Press, 2009.
19. Kurtz SM, Kocagöz SB, Hanzlik JA, et al. Do ceramic femoral heads reduce taper fretting corrosion in hip arthroplasty? A retrieval study. Clin Orthop Relat Res 2013;471(10):3270–3282.
20. Lachiewicz PF, Heckman DS, Soileau ES, et al. Femoral head size and wear of highly cross-linked polyethylene at 5 to 8 years. Clin Orthop Relat Res 2009;467(12):3290–3296.
21. McClung CD, Zahiri CA, Higa JK, et al. Relationship between body mass index and activity in hip or knee arthroplasty patients. J Orthop Res 2000;18(1):35–39.
22. Meftah M, Klingenstein GG, Yun RJ, et al. Long-term performance of ceramic and metal femoral heads on conventional polyethylene in young and active patients: a matched-pair analysis. J Bone Joint Surg Am 2013;95(13):1193–1197.
23. Milošev I, Kovac S, Trebse R, et al. Comparison of ten-year survivorship of hip prostheses with use of conventional polyethylene, metal-on-metal, or ceramic-on-ceramic bearings. J Bone Joint Surg Am 2012;94(19):1756–1763.
24. O’Neill SC, Queally JM, Devitt BM, et al. The role of osteoblasts in peri-prosthetic osteolysis. Bone Joint J 2013;95-B(8):1022–1026.
25. Petsatodis GE, Papadopoulos PP, Papavasiliou KA, et al. Primary cementless total hip arthroplasty with an alumina ceramic-on-ceramic bearing: results after a minimum of twenty years of follow-up. J Bone Joint Surg Am 2010;92(3):639–644.
26. Porat M, Parvizi J, Sharkey PF, et al. Causes of failure of ceramic-on-ceramic and metal-on-metal hip arthroplasties. Clin Orthop Relat Res 2012;470(2):382–387.
27. Ries MD, Link TM. Monitoring and risk of progression of osteolysis after total hip arthroplasty. Instr Course Lect 2013;62:207–214.
28. Roy ME, Whiteside LA, Magill ME, et al. Reduced wear of cross-linked UHMWPE using magnesia-stabilized zirconia femoral heads in a hip simulator. Clin Orthop Relat Res 2011;469(8):2337–2345.
29. Thomas GE, Simpson DJ, Mehmood S, et al. The seven-year wear of highly cross-linked polyethylene in total hip arthroplasty: a double-blind, randomized controlled trial using radiostereometric analysis. J Bone Joint Surg Am 2011;93(8):716–722.
30. Topolovec M, Milošev I. A comparative study of four bearing couples of the same acetabular and femoral component: a mean follow-up of 11.5 years. J Arthroplasty 2014;29(1):176–180.
31. Traina F, Tassinari E, De Fine M, et al. Revision of ceramic hip replacements for fracture of a ceramic component: AAOS exhibit selection. J Bone Joint Surg Am 2011;93(24):e147.
32. Yeung E, Bott PT, Chana R, et al. Mid-term results of third-generation alumina-on-alumina ceramic bearings in cementless total hip arthroplasty: a ten-year minimum follow-up. J Bone Joint Surg Am 2012;94(2):138–144.
33. Wiley KF, Ding K, Stoner JA, et al. Incidence of pseudotumor and acute lymphocytic vasculitis associated lesion (ALVAL) reactions in metal-on-metal hip articulations: a meta-analysis. J Arthroplasty 2013;28(7):1238–1245.
< div class='tao-gold-member'>









