Superficial Cutaneous Infections and Pyodermas: Introduction
|
Normal human skin is colonized soon after birth by a large number of bacteria that live as commensals on the epidermis and epidermal appendages (the skin microbiome). Coagulase-negative Staphylococci (Staphylococcus epidermidis) are inoculated during vaginal passage and coryneform bacteria take up residence on neonatal skin shortly after birth. Within several weeks after birth, the microbiome of neonatal skin is similar to that of adults and includes many species of bacteria and fungi (see Chapter 175).
The majority of the primary and secondary pyodermas (cutaneous bacterial infections) are caused by either S. aureus or group A Streptococcus. These bacteria cause a broad clinical spectrum of infection ranging from superficial pyodermas to invasive soft-tissue infections (STIs; see Chapter 179) depending on the organism, the anatomic location of infections, and on host factors.
Staphylococcal Skin Infections
Staphylococci are classified into two major groups: (1) the coagulase-negative Staphylococci and (2) coagulase-positive (S. aureus) Staphylococci. Individuals carry a minimum of 10–24 combined temporary and resident strains of S. epidermidis, the most common coagulase-negative strain. S. epidermidis is a common colonizer of the skin but is capable of causing superficial and invasive infections (particularly about implants and catheters).
S. aureus permanently colonizes the anterior nares in approximately 20% of the population. Carriage is transient or intermittent in other individuals. Approximately 60% of healthy individuals have occasional carriage of S. aureus at some site.1 Other sites of colonization include the axillae, perineum, pharynx, and hands. Conditions predisposing to S. aureus colonization include atopic dermatitis, diabetes mellitus (insulin dependent), dialysis (hemo- and peritoneal), intravenous drug use, liver dysfunction, and human immunodeficiency virus (HIV) infection. Colonization by S. aureus is found at some body site in up to 37% of patients presenting with purulent community associated methicillin resistant S. aureus (MRSA) infections.2
S. aureus is an aggressive pathogen and the most common cause of primary pyodermas and STIs, as well as of secondary infections on disease-altered skin. S. aureus in pyodermas or STIs can invade the bloodstream, producing bacteremia, metastatic infection such as osteomyelitis, and acute infective endocarditis. Some strains of S. aureus also produce exotoxins, which can cause constellations of cutaneous and systemic symptoms such as staphylococcal scalded-skin syndrome (SSSS) and staphylococcal toxic shock syndrome (TSS).
Transfer of organisms to patients occurs predominantly via the hands of personnel rather than through the air. This appears to be particularly true in newborn nurseries. Any individuals with open staphylococcal infections are high-risk potential carriers and transmitters of infection. Nasal carriage of S. aureus appears to be a major risk factor for wound infection after cardiac surgery, resulting in higher mortality rates and longer postoperative stays.3 The rate of S. aureus bacteremia is also higher in nasal carriers of S. aureus.4 Good nursery technique, careful handling of patients, strict hand-washing procedures, and isolation of patients with open draining staphylococcal infections are important in the reduction of transmission of Staphylococci. In older adults, S. aureus accounts for 9% of nosocomial infections and follows only Escherichia coli, Pseudomonas aeruginosa, and Enterococci in prevalence.5
Colonization by S. aureus may be transient or represent a prolonged carrier state. S. aureus produces many cellular components and extracellular products that may contribute to its pathogenicity. Host factors such as immunosuppression, glucocorticoid therapy, and atopy may play a major role in the pathogenesis of staphylococcal infections. Preexisting tissue injury or inflammation (surgical wound, burn, trauma, dermatitis, retained foreign body) is of major importance in the pathogenesis of staphylococcal disease.
Some strains produce one or more exoproteins, including the staphylococcal enterotoxins (SEA, SEB, SECn, SED, SEE, SEG, SEH, and SEI), and the exfoliative toxins (ETA and ETB), TSS toxin-1 (TSST-1), and leukocidin. These toxins have unique potent effects on immune cells and other biologic effects as well, ultimately inhibiting host immune response.
TSST-1 and the staphylococcal enterotoxins are also known as pyrogenic toxin superantigens. These molecules act by binding directly to constitutively expressed HLA-DR molecules (major histocompatibility complex II) on antigen-presenting cells without antigen processing. Although conventional antigens require recognition by all five elements of the T-cell-receptor complex, superantigens require only the variable region of the β-chain. As a result, 5%–30% of resting T cells may be activated, whereas the “normal” antigenic response is only 0.0001%–0.01% of T cells.6 Nonspecific T-cell activation leads to massive systemic release of cytokines, especially interleukin 2, interferon-γ, and tumor necrosis factor-β from T cells and interleukin 1 and tumor necrosis factor-α from macrophages.7 Superantigen stimulation of T cells also results in activation and expansion of lymphocytes expressing specific T cell-receptor variable region of the β-chain. They may activate B cells, leading to high levels of immunoglobulin E (IgE) or autoantibodies.8 Also, there is evidence that superantigens selectively induce cutaneous lymphocyte-associated antigen on T cells, thereby “homing” them to the skin.8
There are several other mechanisms by which S. aureus evades immune clearance. Approximately 60% of S. aureus strains secrete the chemotaxis inhibitory protein of Staphylococci, which inhibits neutrophil chemotaxis. Additionally, protein A, staphylokinase, capsular polysaccharide, fibrinogen binding protein, and clumping factor A all act to aid in avoidance of being opsonized and phagocytosed. Staphylokinase and aureolysin bind and cleave antimicrobial peptides, respectively, resulting in increased survival in vitro and probably in vivo.1
A major problem in treating staphylococcal infections has been the emergence of antibiotic-resistant strains. With ever-increasing use of penicillins, methicillin-resistant S. aureus (MRSA) strains have become a major epidemiologic problem since the 1980s. Resistance to methicillin indicates pan resistance to all β-lactam antibiotics. Recently, intermediate-level resistance of MRSA to vancomycin has emerged and constitutes a potential further problem in treatment. Although S. aureus infection prevalence has not changed much, the percentage of MRSA isolates of these infections has significantly increased in some countries. Today, MRSA can be divided into Hospital-Associated (HA) or Community-Associated (CA) MRSA. In many areas, the prevalence of CA-MRSA strains is over 50%.9
Attempts to eradicate MRSA have generally been unsuccessful. Treatment of anterior nares and wounds with mupirocin ointment has been shown to decrease S. aureus colonization but in one study did not decrease the rate of transmission to a roommate in a long-term care facility.10 Although there are many other reports of the use of topical mupirocin to reduce colonization of MRSA and methicillin-sensitive S. aureus, indiscriminate use of topical mupirocin must be avoided because significant mupirocin resistance has already emerged.
The primary defense against S. aureus infections is the innate immunity provided by neutrophils.11 A major component of the innate response is antimicrobial peptides. These peptides can be found in various locales and include dermicidin, LL-37, protegrin, α-defensins and β-defensins, lactoferricin, and cascocidin. However, S. aureus also has the ability to thwart the immune system in several ways. One of the main virulence factors of S. aureus is the production of adhesins that facilitate binding to host epithelial cell surfaces. The almost universal presence in adults of circulating antibodies to one or more cell-wall antigens or extracellular toxins substantiates the high prevalence of staphylococcal infections. However, these antibodies are not the primary determinants of resistance to such infections. Recent evidence demonstrates that S. aureus can invade and survive in many types of host cells, suggesting that a cell-mediated immune response may be required for killing intracellular organisms.
Pyodermas are infections in the epidermis, just below the stratum corneum or in hair follicles. In industrialized nations, S. aureus is the most common cause of superficial pyodermas (Box 176-1), but group A Streptococcus continues to be a common cause of pyoderma in developing countries. If untreated, pyodermas can extend to the dermis, resulting in ecthyma and furuncle formation.
SITES OF COLONIZATION (CARRIER STATE)
|
SITES OF COLONIZATION IN NEONATES (AND SITES OF INFECTION)
|
SUPERFICIAL PYODERMAS
|
INVASIVE INFECTIONS
|
METASTATIC SKIN INFECTIONS ASSOCIATED WITH BACTEREMIA (OFTEN S. aureus ACUTE INFECTIONS ENDOCARDITIS)
|
PURPURA FULMINANS
|
STAPHYLOCOCCAL TOXIN-ASSOCIATED SYNDROMES
|
Two clinical patterns of impetigo are recognized: (1) bullous and (2) nonbullous. Bullous impetigo is caused by S. aureus. Currently, in industrialized nations, nonbullous impetigo is most commonly caused by S. aureus and less often by group A Streptococcus. Group A Streptococcus remains a common cause of nonbullous impetigo in developing nations.
The nonbullous type of impetigo accounts for more than 70% of cases of this form of pyoderma. It occurs in children of all ages as well as in adults. Intact skin is usually resistant to colonization or impetiginization, possibly due to absence of fibronectin receptors for teichoic acid moieties on S. aureus and group A Streptococcus. Production of bacteriocins, produced by certain S. aureus strains (phage group 71) and highly bactericidal to group A Streptococcus, may be responsible for the isolation of only S. aureus from some lesions initially caused by Streptococci.
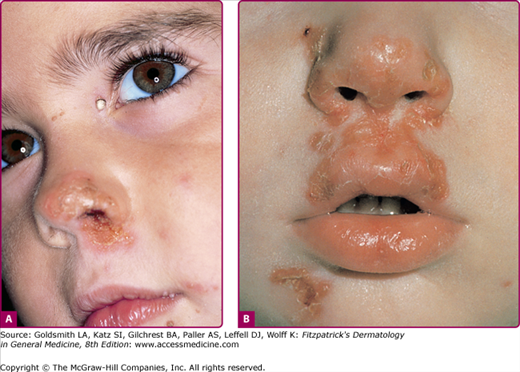
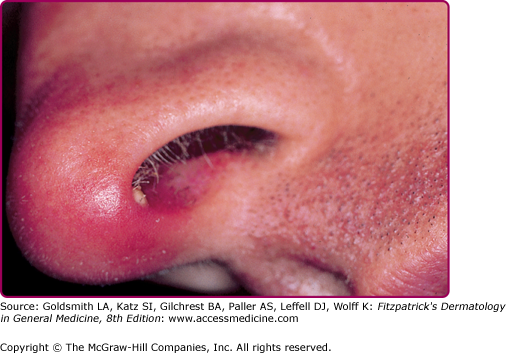
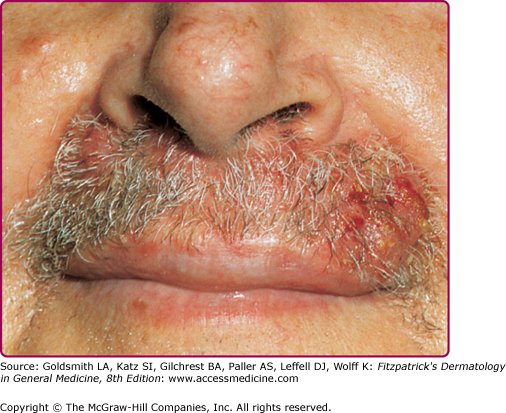
In a typical sequence, S. aureus spreads from nose to normal skin (approximately 11 days later) and then develop into skin lesions (after another 11 days). Lesions commonly arise on the skin of the face (especially around the nares) or extremities after trauma. Nasal carriers of S. aureus can present with a very localized type of impetigo confined to the anterior nares and the adjacent lip area (Fig. 176-1); pruritus or soreness of the area is a common complaint (Fig. 176-2). Conditions that disrupt the integrity of the epidermis, providing a portal of entry of impetiginization, include insect bites, epidermal dermatophytoses, herpes simplex, varicella, abrasions, lacerations, and thermal burns.
See Box 176-2 for differential diagnosis of nonbullous impetigo.
Consider
|
Always Rule Out
|
The initial lesion is a transient vesicle or pustule (see Fig. 176-2) that quickly evolves into a honey-colored crusted plaque that can enlarge to greater than 2 cm in diameter (see Fig. 176-1). Surrounding erythema may be present. Constitutional symptoms are absent. Regional lymphadenopathy may be present in up to 90% of patients with prolonged, untreated infection. If untreated, the lesions may slowly enlarge and involve new sites over several weeks. In some individuals, lesions resolve spontaneously; in others, the lesions extend into the dermis, forming an ulcer (see Section “Staphylococcal Ecthyma”).
Three types of skin eruptions can be produced by phage group II S. aureus, particularly strains 77 and 55: (1) bullous impetigo, (2) exfoliative disease (SSSS), and (3) nonstreptococcal scarlatiniform eruption (staphylococcal scarlet fever). All three represent varying cutaneous responses to extracellular exfoliative toxins (“exfoliatin”) types A and B produced by these Staphylococci (see Chapter 177). Exfoliative toxin A acts as a serine protease of desmoglein 1, the desmosomal cadherin that is also the target of autoantibodies in pemphigus foliaceus.12
In a study of bullous impetigo, 51% of patients had concurrent S. aureus cultured from the nose or throat, and 79% of cultures grew the same strain from both sites.
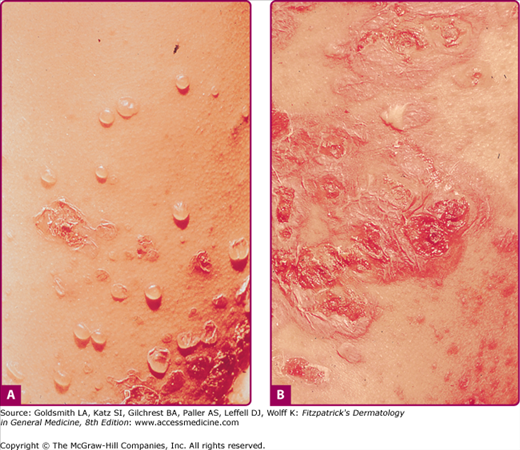
Bullous impetigo occurs more commonly in the newborn and in older infants, and is characterized by the rapid progression of vesicles to flaccid bullae (Fig. 176-3). Decades ago, extensive bullous impetigo (archaic term: pemphigus neonatorum or Ritter disease) occurred in epidemics within neonatal nurseries.
Bullae usually arise on areas of grossly normal skin. The Nikolsky sign (sheet-like removal of epidermis by shearing pressure) is not present. Bullae initially contain clear yellow fluid that subsequently becomes dark yellow and turbid (see Fig. 176-3A), and their margins are sharply demarcated without an erythematous halo. The bullae are superficial, and within a day or two, they rupture and collapse, at times forming thin, light-brown to golden-yellow crusts (see Fig. 176-3B). So-called bullous varicella represents superinfection by S. aureus (phage group II) of varicella lesions (bullous impetiginization).
Gram stain of exudates from bullous impetigo reveals Gram-positive cocci in clusters. S. aureus belonging to phage group II can be cultured from the contents of intact bullae.
Histologically, the lesions of bullous impetigo show vesicle formation in the subcorneal or granular region, occasional acantholytic cells within the blister, spongiosis, edema of the papillary dermis, and a mixed infiltrate of lymphocytes and neutrophils around blood vessels of the superficial plexus.
See Box 176-3 for differential diagnosis of bullous impetigo.
Consider
|
Always Rule Out
|
If untreated, invasive infection can complicate S. aureus impetigo with cellulitis, lymphangitis, and bacteremia, resulting in osteomyelitis, septic arthritis, pneumonitis, and septicemia. Exfoliatin production can lead to SSSS in infants and in adults who are immunocompromised or have impaired renal function.
See Box 176-4. Local treatment with mupirocin ointment or cream, removal of crusts, and good hygiene is sufficient to cure most mild to moderate cases.13 Retapamulin 1% ointment is also effective for localized impetigo and secondarily impetiginized dermatitis as well, although decreased efficacy against MRSA was noted in some trials.14 Fusidic acid is an equally effective topical agent for localized impetigo and has very few adverse effects topically. However, it is currently unavailable in the United States.15 Systemic antibiotics may be required in extensive cases. The frequency of isolation of group A Streptococcus makes such therapy a reasonable approach in most patients who have a significant degree of involvement. There is no role for general disinfectant treatments or bacitracin.16
Topical | Systemic | |||
|---|---|---|---|---|
First line | Mupirocin Retapamulin Fusidic acid (not available in United States) | bid bid bid | Dicloxacillin Amoxicillin plus clavulanic acid; cephalexin | 250–500 mg PO qid for 5–7 days 25 mg/kg tid; 250–500 mg qid |
Second line (penicillin allergy) | Azithromycin Clindamycin Erythromycin | 500 mg × 1, then 250 mg daily for 4 days 15 mg/kg/day tid 250–500 mg PO qid for 5–7 days | ||
If CA-MRSA is suspected | Mupirocin | bid | TMP-SMX Clindamycin Tetracycline Doxycycline, Minocycline | 160/800 mg PO bid for 7 days 15 mg/kg/day tid 250–500 mg PO qid for 7 days 100 mg PO bid for 7 days |
Staphylococcal impetigo responds quite promptly to appropriate treatment. In an adult with extensive or bullous lesions, dicloxacillin (or similar penicillinase-resistant semisynthetic penicillin), 250–500 mg orally (PO) four times daily (qid), or erythromycin (in the penicillin-allergic patient), 250–500 mg PO qid, should be given. Treatment should be continued for 5–7 days (10 days if Streptococci are isolated). Also, a single course of oral azithromycin (in adults 500 mg on the first day, 250 mg daily on the next 4 days) has been shown to be equally as effective as dicloxacillin for skin infections in adults and children. For impetigo caused by erythromycin-resistant S. aureus, which is commonly isolated from impetigo lesions of children, amoxicillin plus clavulanic acid [25 mg/kg/day given three times a day (tid)], cephalexin (40–50 mg/kg/day), cefaclor (20 mg/kg/day given tid), cefprozil (20 mg/kg once daily), or clindamycin (15 mg/kg/day tid or qid) given for 10 days are effective alternative therapies. If CA-MRSA is likely, consider the following therapies: TMP-SMX and rifampin (100%), clindamycin (95%), and tetracycline (92%). Inducible resistance to clindamycin should be excluded by performing a D-zone disk-diffusion test.
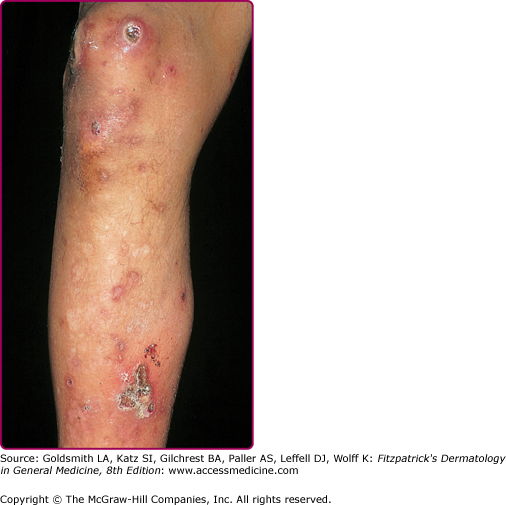
Ecthyma is a cutaneous pyoderma characterized by thickly crusted erosions or ulcerations. Ecthyma is usually a consequence of neglected impetigo and classically evolves in impetigo occluded by footwear and clothing. Thus, it is a lesion, typically occurring in the homeless and soldiers in combat on maneuver in a humid and hot climate. S. aureus and/or group A Streptococcus can be isolated on culture. Untreated staphylococcal or streptococcal impetigo can extend more deeply, penetrating the epidermis, producing a shallow crusted ulcer (Fig. 176-4). Ecthymatous lesions can evolve from a primary pyoderma or within a preexisting dermatosis or site of trauma. Ecthyma gangrenosum is a cutaneous ulcer caused by P. aeruginosa and resembles staphylococcal or streptococcal ecthyma (see Chapter 180).
Ecthyma occurs most commonly on the lower extremities of children, or neglected elderly patients, or individuals with diabetes. Poor hygiene and neglect are key elements in pathogenesis.
The ulcer has a “punched-out” appearance when the dirty grayish-yellow crust and purulent material are debrided. The margin of the ulcer is indurated, raised, and violaceous (see Fig. 176-4), and the granulating base extends deeply into the dermis. Untreated ecthymatous lesions enlarge over weeks to months to a diameter of 2–3 cm or more.
The lesions are slow to heal, requiring several weeks of antibiotic treatment for resolution. Problems of spread by autoinoculation or by insect vectors and of poststreptococcal sequela (glomerulonephritis) are the same as with impetigo.
Management of ecthyma is usually systemic and includes the same agents used for staphylococcal impetigo (see Box 176-4).
Folliculitis is a pyoderma that begins within the hair follicle, and is classified according to the depth of invasion (superficial and deep), and microbial etiology (Box 176-5).
BACTERIAL FOLLICULITIS
|
FUNGAL FOLLICULITIS
|
VIRAL FOLLICULITIS
|
INFESTATION
|
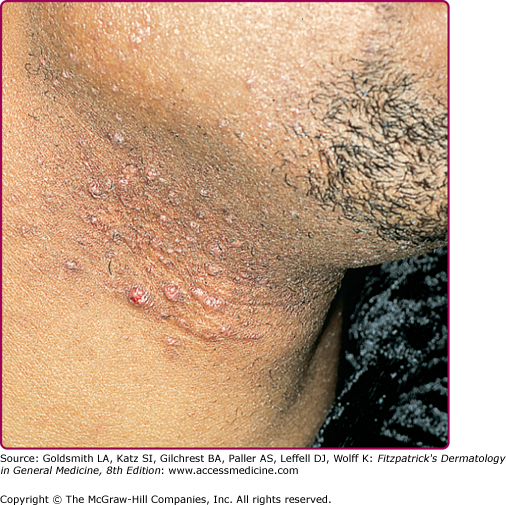
Superficial folliculitis has also been termed follicular or Bockhart impetigo. A small, fragile, dome-shaped pustule occurs at the infundibulum (ostium or opening) of a hair follicle, often on the scalps of children and in the beard area (Fig. 176-5), axillae, extremities, and buttocks of adults. Isolated staphylococcal folliculitis is common on the buttock of adults. Periporitis staphylogenes refers to secondary infection of miliaria of the neonate by S. aureus. Staphylococcal blepharitis is an S. aureus infection of the eyelids, presenting with scaling or crusting of the eyelid margins, often with associated conjunctivitis; the differential diagnosis includes seborrheic dermatitis and rosacea of the eyelid.
S. aureus folliculitis must be differentiated from other folliculocentric infections. Also, three noninfectious, inflammatory, follicular disorders are more common in black men: (1) pseudofolliculitis barbae, which occurs on the lower beard area (Fig. 176-6); (2) folliculitis keloidalis or acne keloidalis nuchae, on the nape of the neck; and (3) perifolliculitis capitis, on the scalp. S. aureus can cause secondary infection in these inflammatory disorders. Exposure to mineral oils, tar products, and cutting oils can cause an irritant folliculitis. Acne vulgaris, drug-induced acneiform eruptions, rosacea, hidradenitis suppurativa, acne necrotica of the scalp, and eosinophilic folliculitis of HIV disease must be distinguished from infectious folliculitis as well. Also, “hot tub” folliculitis may be caused by P. aeruginosa (see Chapter 180).
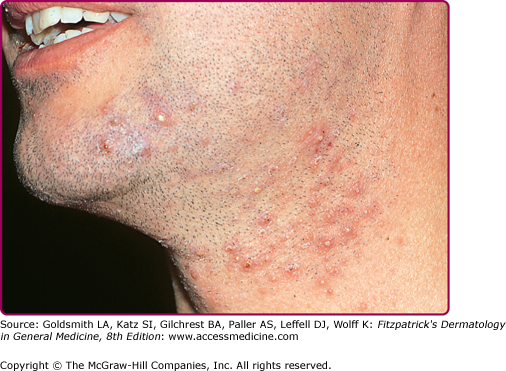
Sycosis barbae is a deep folliculitis with perifollicular inflammation occurring in the bearded areas of the face and upper lip (Fig. 176-7). If untreated, the lesions may become more deeply seated and chronic. Local treatment with warm saline compresses and local antibiotics (mupirocin or topical clindamycin) may be sufficient to control infection. More extensive cases require systemic antibiotic therapy. Dermatophytic folliculitis must be differentiated from S. aureus folliculitis. In fungal infections, hairs are usually broken or loosened, and there are suppurative or granulomatous nodules rather than pustules. Also, in dermatophytic folliculitis plucking of hairs is usually painless (see Chapter 188).
Lupoid sycosis is a deep, chronic form of sycosis barbae associated with scarring, usually occurring as a circinate lesion. A central cicatrix surrounded by pustules and papules gives the appearance of lupus vulgaris (see Chapter 184).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree