Stem cell technology has been discussed chiefly in terms of organ replacement in end-stage diseases. However, improved understanding of adult stem cells and a more nuanced appreciation of aging skin as a disease state has focused greater attention on the potential for truly regenerative and rejuvenative skin therapy with autologous cells. Through enhanced understanding of the normal processes of wound healing, systems of treatment and avenues of therapy are emerging based on modulation and amplification of the natural processes of wound healing. This article presents skin-specific developments in stem cell and growth factor science and suggests further avenues of investigation.
Key points
- •
Stem cells are capable of self-replication and differentiation into various cell lines. Embryonic stem cells can differentiate into all cells lines, whereas adult stem cells can differentiate into cells types within their cell line. Treatment with certain transcription factors can induce adult cells to revert to induced pleuripotent stem cells.
- •
Mesenchymal stem cells tolerate ischemia and respond by replication.
- •
Adipose-derived stem cells (ADSCs) can affect local tissues both through differentiation and release of growth factors.
- •
ADSCs can stimulate the growth of existing capillaries and the development of new capillaries.
- •
Platelet-rich plasma and platelet-rich fibrin matrix, through the release of platelet growth factors, can induce local cellular changes.
- •
ADSCs and platelet preparations have been described for use in aesthetic surgery; however, optimal delivery and application is still being defined.
Clinical review of the literature
Restoration of the skin’s normal architecture, contours, and physical characteristics is currently addressed with a variety of chemical, mechanical, and energy-based technologies. However, beyond the concept of wound contracture followed by wound healing, little is currently known about the process. At best, created wounds will heal naturally.
As our understanding of the cellular and biochemical milieu of the skin wound becomes more robust and thorough, attempts to enhance the clinical response to wounding based on these laboratory findings have been made; however, these are often shotgun approaches, and interpreting the results of some of these studies can be challenging and difficult to reconcile.
A more thorough grasp of the nature of autologous stem cells and the growth factors to which they respond and secrete is now emerging, providing a framework on which more rational treatment strategies can be built.
Classic wound healing occurs in 3 overlapping phases: the inflammatory, proliferative, and remodeling phases. During the inflammatory phase, platelets aggregate on a fibrin clot and achieve active hemostasis, whereas acute inflammatory cells remove debris and create a favorable environment for the proliferative phase to occur. The proliferative phase is characterized by the formation of granulation tissue, collagen deposition, angiogenesis, epithelial migration, and wound coverage. Remodeling occurs with collagen resorption and replacement.
However, it is now known that fibrin does more than serve as a binding site for platelets. The fibrin mesh that forms acts as scaffolding for subsequent cell binding and cellular activity (including collagen deposition), and provides binding sites for growth factors. A principal source of growth factors is the platelet, which releases stored growth factors from alpha granules on activation and continues to synthesize and secrete these growth factors for the rest of the lifespan of the platelets (5–10 days). These growth factors are chemoattractive, mitogenic, and angiogenic; of these, vascular endothelial growth factor (VEGF) and transforming growth factor-beta (TGF-β) are the best characterized.
During the proliferative phase, collagen is deposited, new blood vessels are formed and epithelial continuity is reestablished. Neovascularization occurs both by angiogenesis (blood vessel development from existing capillaries) and vasculogenesis (generation of new capillaries de novo). Vasculogenesis requires the presence of stem cells, which serve not only as progenitor cells but also regulate tissue growth through the release of growth factors. Resident tissue-specific stem cells in the hair follicle bulge out of the outer root sheath and in the basal layer of the interfollicular epidermis, they respond to injury by expansion to reepithelialize the wound.
Stem cells are characterized by their ability to replicate as well as differentiate under specific conditions into specific cell lines. Pluripotent cells, such as embryonic stem cells, are capable of differentiating into every cell in the body, whereas mesenchymal stem cells (MSCs), such as bone-marrow-derived (BMDSCs) or adipose-derived stem cells (ADSCs), can differentiate into cells in the mesenchymal line. ADSCs are an attractive source of MSCs as the fat can be easily harvested via a liposuction procedure. Differential centrifugation isolates the stromal vascular fraction (SVF), containing white and red blood cells, endothelial cells, fibroblasts, pericytes, and ADSCs. Jurgens and colleagues have argued that abdominal fat harvesting yields higher SVF and ADSC cell counts than other donor areas, although several other studies have failed to demonstrate significant differences in recovery of viable adipocytes from various donor sites. Although the SVF can be added to adipocytes and injected in the technique of cell-assisted lipotransfer (CAL), ADSCs can also be isolated and expanded in vitro before use.
MSCs, such as ADSCs, possess many interesting and exploitable properties in terms of wound healing. In addition to their capacity for self-renewal and differentiation, MSCs are known to better tolerate ischemia and proliferate under ischemic conditions. MSCs migrate to areas of ischemia, injury and inflammation. MSCs secrete a variety of growth factors, including VEGF, TGF-β, hepatocyte growth factor, platelet-derived growth factor (PDGF), basic fibroblast growth factor, and placental growth factor, and stimulate a proangiogenic, immunotolerant, and antioxidative environment through cytokine release. Analysis of critical length skin flaps treated with injections of radiolabeled ADSCs demonstrated that, in addition to the proangiogenetic effects mediated by VEGF and PDGF (among other growth factors), new capillaries are formed directly from the ADSCs. However, by virtue of the numbers of cells involved as well as the positive effect seen with the application of MSC culture media, it is clear that a significant degree of MSC activity is via a paracrine effect.
Numerous animal studies have been performed to examine the effect of MSCs/ADSCs on wound healing, and have included application of stem cells topically or by local injection. Steinberg and colleagues found no difference in healing of ischemic wounds between groups treated with topically applied ADSCs or dermal fibroblasts in fibrin sealant, but many studies have shown a positive therapeutic effect. Clinically, there are number of case reports describing stem cell use, including a report by Rigotti and colleagues of subjective improvement in 20 patients with severe radiodermatitis and osteoradionecrosis treated with ADSCs. CAL as described by Yoshimura is probably the best known application of stem cells in aesthetic plastic surgery.
Although stem cells can provide both the cellular proliferative engines and paracrine stimuli to enhance wound healing, platelets can provide the necessary stimuli for the wound-healing response through the release of growth factors. Topical PDGF (becaplermin 0.01%, Regranex, OMJ Pharmaceuticals, Inc, San German, Puerto Rico) and keratinocyte growth factor (KGF, palifermin, Kepivance, Biovitrum AB, Stockholm, Sweden) are approved by the US Food and Drug Administration (FDA) for diabetic foot ulcers and oral mucositis, respectively, but prolonged use has been associated with an increase in cancer mortality.
Platelet-rich plasma (PRP) seeks to amplify the body’s normal wound response. PRP has traditionally been defined as a preparation with at least a 5- to 6-fold increase in platelet concentration (compared with peripheral blood) in plasma; fibrin is not concentrated and limited due to the low plasma volume. Platelets are activated with bovine thrombin and (usually) supraphysiologic concentrations of calcium. Red blood cells are generally limited, but white cells are generally increased. An alternative preparation, platelet-rich fibrin matrix (PRFM), is a less concentrated platelet preparation, but includes plasma proteins, especially fibrinogen/fibrin, providing a more physiologic milieu for platelet function. PRFM has been shown to release bioactive platelet growth factors for up to 7 days after activation.
PRP has been shown to accelerate experimental as well as chronic nonhealing wounds. The cellular effects of PRP seem to occur early and be short-lived. However, the stimulation of cells can begin a reparative process. PRP injections into ischemic skin flaps decrease skin necrosis and are associated with increased mRNA for VEGF, PDGF, and endothelial growth factor.
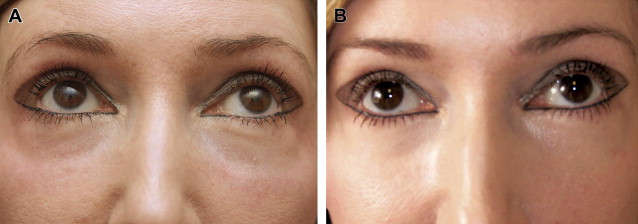
PRFM has also been shown to be effective in chronic nonhealing lower extremity ulcers. Sclafani first described the aesthetic use of PRFM in 2010, demonstrating significant improvement in deep nasolabial folds in as little as 2 weeks and persisting for 12 weeks (the duration of the study) after dermal and subdermal injections of PRFM. Histologically, intradermal PRFM injection is associated with activation of fibroblasts with collagen deposition, development of new blood vessels, and adipogenesis. PRFM has been used in a variety of applications in aesthetic facial plastic surgery ( Fig. 1 ), both by dermal and subdermal injection or mixed with autologous fat before injection. In addition to the effects of the growth factors released from the platelets, fibrin mesh binding of the harvested fat lobules may provide additional structural integrity to support the clinical observation of enhanced fat survival when mixed with PRFM. This result contrasts with the mixed results of PRP treatment of fat grafts.