INTRODUCTION
I. EPIDEMIOLOGY
A. Squamous cell carcinoma (SCC) is the most common cancer involving head and neck mucosal sites (accounts for 90% of malignancies)
B. Most often occurs in the sixth and seventh decades of life
C. Incidence increases with age
D. Male to female ratio is approximately 2:1
E. Risk factors
1. Tobacco (including smokeless/chewing tobacco)
2. Alcohol: Synergizes with tobacco; increases risk by 10- to 15-fold
3. Human papilloma virus (HPV): Responsible for increasing incidence of oropharyngeal SCC
4. Epstein–Barr virus (EBV): Associated with nasopharyngeal SCC
5. Poor dental hygiene
6. Chronic irritation (i.e., ill-fitting dentures)
7. Plummer–Vinson syndrome (achlorhydria, iron deficiency anemia, dysphagia, mucosal atrophy)
8. Syphilis
9. Lichen planus
10. Chronic immunosuppression
11. Betel nuts: Common in Indian population
II. PATHOLOGY
A. Premalignant lesions
1. Require close follow-up; biopsy required to rule out invasive component
2. Leukoplakia
a. Clinical description of white patchy mucosa
b. Represents epithelial hyperplasia, usually secondary to trauma
c. May harbor dysplasia, carcinoma in situ, or invasive SCC
3. Erythroplakia: Clinical description of red “velvet-like” mucosal patches
a. Higher incidence of associated SCCA compared to leukoplakia
b. Biopsy required to rule out SCCA
4. Lichen planus
a. White, flat inflammatory papule involving oral mucosa
b. 5% undergoes malignant transformation
B. Gross variants of SCCA
1. Ulcerative type: Most common type of oral cavity SCCA
2. Infiltrative type
a. Often found in tongue SCCA
b. Diagnosis requires careful palpation
3. Exophytic type
a. Spreads superficially
b. Less aggressive/less likely to metastasize
C. SCCA histology
1. Three histologic variants (well, moderately, and poorly differentiated)
______________
*Denotes common in-service examination topics
2. Well-differentiated lesions have increased amounts of keratin and predict a better prognosis.
3. Nasopharyngeal carcinoma has a separate World Health Organization (WHO) classification.
a. WHO type I: SCCA; represents 25% of nasopharyngeal carcinoma
i. Not associated with EBV
ii. Worse prognosis
iii. Less sensitive to radiation
b. WHO type II: nonkeratinizing; 12% tumors
i. Associated with EBV
ii. Better prognosis
iii. Sensitive to radiation
c. WHO type III: Undifferentiated (includes lymphoepithelioma, anaplastic and clear cell variants); >60% tumors
i. Associated with EBV
ii. Better prognosis
iii. Sensitive to radiation
4. Verrucous carcinoma (Ackerman’s tumor)
a. Rare variant of SCCA
b. Most often involves buccal mucosa followed by gingival mucosa
c. Exophytic; papillary morphology
d. Deep infiltration and metastasis uncommon
e. Treatment
i. Surgical excision with only a few millimeters of margin
ii. Serial sectioning of pathology specimen necessary to rule out harboring invasive SCCA.
iii. Role of radiation controversial due to potential for transformation into an anaplastic/aggressive lesion.
D. Metastatic disease
1. Regional spread to cervical lymph nodes
a. Follows a predictable pattern depending on location of primary tumor.
b. Midline tumors can drain to bilateral nodal basins.
c. Poor prognostic factors include multiple lymph node involvement, presence of extracapsular spread (ECS), perineural invasion, and matted nodes.
2. Distant metastasis is most often to lung, and sometimes to bone.
III. ANATOMY
A. Oral cavity
1. Extends from the skin-vermilion lip junction posteriorly to the junction of the hard and soft palate and circumvallate papillae.
2. The oral cavity is divided into a number of subsites
a. Lips
b. Buccal mucosa
c. Upper and lower alveolar ridge
d. Retromolar trigone (RMT)
e. Floor of mouth (FOM)
f. Hard palate
g. Anterior two-third of tongue
B. The pharynx is divided into three subsites
1. Nasopharynx: Extends from the skull base superiorly to the soft and hard palate inferiorly; from the nasal choanae/septum to the posterior pharyngeal wall. Subsites of the nasopharynx include:
a. Fossa of Rosenmuller
b. Torus and orifice of the Eustachian tube
c. Lateral and posterior walls
2. Oropharynx
a. Anterior border: Circumvallate papillae
b. Lateral borders: Tonsil, tonsillar fossa, and tonsillar pillars
c. Posterior border: Posterior pharyngeal wall
d. Inferior border: Floor of vallecula (space between base of tongue (BOT) and epiglottis)
e. Superior border: Soft palate
f. Weldeyer’s ring, within the oropharynx, includes lymphoid tissue of the palatine and lingual tonsils
3. Hypopharynx: Extends from floor of vallecula and aryepiglottic folds to inferior border of cricoid cartilage; contains three subsites.
a. Pyriform sinuses
b. Posterior pharyngeal wall
c. Postcricoid region/pharyngoesophageal junction (least common)
C. Larynx: Three subsites
1. Supraglottis (30% of laryngeal SCCA)
a. Separated from glottis by horizontal plane through ventricle (space between true and false vocal cords).
b. Includes epiglottis, aryepiglottic folds, arytenoids, false vocal cords, and ventricles.
2. Glottis (50% to 70% of laryngeal SCCA)
a. Extends from ventricle to 1 cm below the free edge of the true vocal cord.
b. Includes true vocal cords, anterior, and posterior commissure.
c. “Transglottic” tumors cross glottis in continuity with another site
3. Subglottis: Extends from glottis to inferior border of cricoid ring.
D. Other spaces
1. Pre-epiglottic space
a. Bound superiorly by the hyoepiglottic ligament and vallecula, anteriorly by the thyrohyoid ligament, and posteriorly by the epiglottis and thyroepiglottic ligament.
b. The infrahyoid epiglottis has numerous holes in the cartilage allowing cancer to easily spread from the larynx to this space.
2. Paraglottic space
a. Potential space between the thyroid cartilage and medial mucosa wall of the pyriform sinus.
b. Continuous with pre-epiglottic space anteriorly and superiorly.
3. Reinke’s space: Between true vocal cord epithelium and thyroarytenoid muscle.
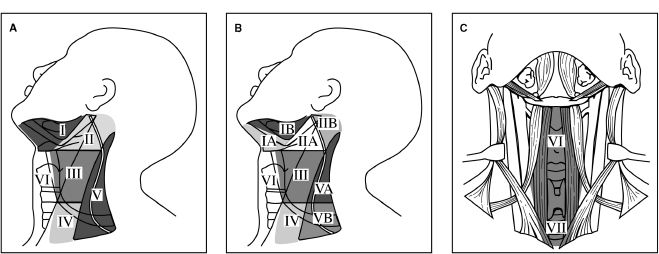
E. Lymphatic drainage levels of the neck (Fig. 16-1)
1. Level I (submental/submandibular triangle): Bound by anterior and posterior digastric muscles, inferior hyoid bone, and body of the mandible.
2. Level II (upper jugular): Bound by skull base, inferior border of hyoid, stylohyoid muscle, and lateral border of sternocleidomastoid muscle (SCM).
Figure 16-1. Lymphatic drainage levels of the neck. Level I, submental and submandibular lymph node groups; level II, upper jugular group; level III, middle jugular groups; level IV, lower jugular group; level V, posterior triangle group; level VI, anterior compartment group. (From Fischer JF, ed. Mastery of Surgery. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2012.)
3. Level III (mid-jugular): Bound by inferior border of hyoid, inferior border of cricoid cartilage, lateral border of sternohyoid, and lateral SCM.
4. Level IV (lower jugular): Bound by inferior boarder of cricoid cartilage, clavicle, lateral border of sternohyoid muscle, and lateral border of SCM.
5. Level V (posterior triangle): Bound by apex of SCM and trapezius muscle, clavicle, posterior border of SCM, and anterior border of trapezius muscle.
6. Level VI (upper mediastinum): Bound by the hyoid bone, suprasternal notch, and common carotid arteries.
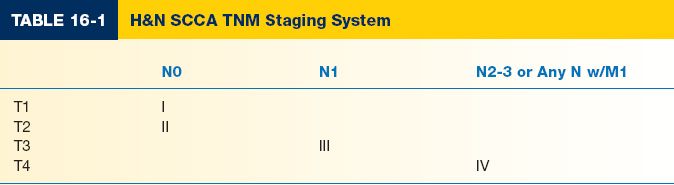
IV. TUMOR, NODE, METASTASIS (TNM) STAGING SYSTEM
A. Clinical staging system
1. Stage denoted by Roman numeral (Table 16-1)
2. Takes into account T, N, and M levels to give a clinical stage
3. Treatment and prognosis are determined by stage
B. Primary tumor (T)
1. Oral cavity (OC)
a. TX: Cannot assess
b. T0: No evidence of tumor
c. Tis: Carcinoma in situ
d. T1: ≤2 cm
e. T2: >2 cm and ≤4 cm
f. T3: >4 cm
g. T4a: Invades adjacent structures (cortical bone, extrinsic tongue musculature, inferior alveolar nerve, floor of mouth, maxillary sinus, facial skin).
h. T4b: Invades masticator space, pterygoid plates, skull base, or encases internal carotid artery.
2. Oropharynx (OP)
a. T1: ≤2 cm
b. T2: >2 cm and ≤4 cm
c. T3: >4 cm
d. T4a: Invades larynx, extrinsic tongue musculature, medial pterygoid muscle, hard plate, or mandible
e. T4b: Invades lateral pterygoid muscle, pterygoid plate, lateral nasopharynx, skull base, or encases carotid artery
3. Hypopharynx (HP)
a. T1: Tumor limited to 1 subsite or ≤2 cm
b. T2: Tumor involves more than 1 subsite or is >2 cm (and ≤4 cm) without vocal cord fixation
c. T3: >4 cm or vocal cord fixation or extension into esophagus
d. T4a: Invades thyroid/cricoid cartilage, hyoid bone, thyroid gland, esophagus, strap muscles/subcutaneous tissue
e. T4b: Invades prevertebral fascia, mediastinum, or encases carotid artery
4. Nasopharynx (NP)
a. T1: Confined to nasopharynx
b. T2a: Extends to soft tissues of OP/nasal cavity without parapharyngeal extension
c. T2b: Any tumor with parapharyngeal extension.
d. T3: Involves bony structures or paranasal sinuses.
e. T4: Intracranial extension, cranial nerve involvement, infratemporal fossa, hypopharynx, orbit, or masticator space.
5. Supraglottis
a. T1: Limited to one subsite with normal vocal cord movement.
b. T2: Invades mucosa of more than one adjacent supraglottic or glottic subsite or region outside supraglottis (BOT, vallecula, medial wall of pyriform sinus) without vocal cord fixation.
c. T3: Tumor limited to larynx with vocal cord fixation or invasion of postcricoid area, pre-epiglottic space, paraglottic space, or inner cortex thyroid cartilage.
d. T4a: Invasion through thyroid cartilage or invasion beyond larynx (i.e., trachea, deep extrinsic tongue musculature, strap muscles, thyroid gland, or esophagus).
e. T4b: Invades prevertebral space, mediastinum, or encases carotid artery
6. Glottis
a. T1a: Limited to one vocal cord with normal mobility.
b. T1b: Involves both vocal cords with normal mobility.
c. T2: Extension to supraglottis or subglottis or impaired vocal cord mobility.
d. T3: Tumor limited to larynx with vocal cord fixation; invasion of paraglottic space or inner cortex thyroid cartilage.
e. T4a: Invasion through thyroid cartilage or invasion beyond larynx (i.e., trachea, deep extrinsic tongue musculature, strap muscles, thyroid gland, or esophagus).
f. T4b: Invades prevertebral space, mediastinum, or encases carotid artery.
7. Subglottis
a. T1: Limited to subglottis
b. T2: Extends to vocal cord(s) with normal or impaired mobility
c. T3: Limited to larynx with vocal cord fixation
d. T4a: Invasion through thyroid cartilage or invasion beyond larynx (i.e., trachea, deep extrinsic tongue musculature, strap muscles, thyroid gland, or esophagus)
e. T4b: Invades prevertebral space, mediastinum, or encases carotid artery
C. Regional lymph nodes (N)
1. Nx: Cannot assess
2. N0: No regional involvement
3. N1: Ipsilateral lymph node ≤3 cm
4. N2a: Single ipsilateral lymph node >3 cm but ≤6 cm
5. N2b: Multiple ipsilateral lymph nodes all ≤6 cm
6. N2c: Bilateral or contralateral lymph node ≤6 cm
7. N3: Lymph node >6 cm
D. Distant metastasis (M)
1. Mx: Cannot assess
2. M0: No distant metastasis
3. M1: Distant metastasis
EVALUATION
I. HISTORY
A. Duration of lesion or mass, and rapidity of enlargement, should be determined
B. Associated symptoms may include
1. Localized pain
2. Odynophagia (painful swallowing)
3. Otalgia (referred ear pain)
4. Hoarseness (indicating glottic involvement)
5. Dysphagia (difficulty swallowing)
6. Weight loss
7. Shortness of breath/stridor
8. Hemoptysis
1. Tobacco use (type; number of years)
2. Alcohol (type; daily amount consumed)—patient may require prophylaxis with benzodiazepines to prevent delirium tremors (DTs) if hospitalization planned
D. Past medical history
1. Past history of head and neck SCCA
2. Previous exposure to radiation
II. PHYSICAL EXAM
A. Tympanic membranes: Middle ear effusion may indicate nasopharyngeal mass
B. Oral cavity
1. State of dentition is important for radiation and reconstructive considerations. Teeth may need to be extracted if they have excessive caries prior to radiation therapy (post-XRT extraction can be inciting event in osteoradionecrosis).
2. Note size and location of suspicious lesions.
3. Comment on fixation of lesion to surrounding bone.
4. Describe extension of tumor by noting all structures involved
5. Deviation of tongue on protrusion indicates involvement of hypoglossal nerve (CN XII) ipsilateral to the deviation.
6. Trismus (inability to fully open mouth) indicates possible involvement of pterygoid muscle, masseter muscle, and/or infratemporal fossa.
C. Oropharynx
1. Note size and location of suspicious lesions
2. Comment on fixation to surrounding bone
3. Describe extension of tumor
4. Palpate BOT and RMT because lesions can infiltrate and/or be difficult to visualize.
D. Larynx
1. Perform indirect examination with mirror visualization.
2. Direct visualization with flexible laryngoscopy should be performed.
3. Assess airway, nasal portion of the soft palate, vocal cord mobility, pyriform sinuses, epiglottis, and vallecula.
4. Anticipate potential need for surgical airway prior to treatment.
E. Neck
1. Careful palpation for cervical lymphadenopathy is performed.
a. Comment on node size, location, and fixation.
b. “Lymph nodes” greater than 3 cm are likely matted nodes.
2. A neck mass can also represent direct tumor extension.
3. Fixation of the larynx (loss of laryngeal crepitus and ability to move larynx side-to-side) is indicative of extralaryngeal tumor extension.
III. LABORATORY STUDIES
A. CBC
B. Coagulation studies (PT, PTT)
C. Electrolyte panel
D. Liver enzymes, including alkaline phosphatase
IV. RADIOGRAPHIC STUDIES
A. CT scan of the neck with contrast (axial and coronal)
1. Evaluate tumor extension
2. Assess bony and cartilaginous invasion
3. Evaluate cervical lymph node involvement
4. Evaluate great vessel involvement/encasement
B. MRI is helpful in evaluating skull base involvement and neural invasion
C. Panorex is useful for evaluating mandibular bone involvement if the CT scan is equivocal
D. Chest X-ray is used to screen for pulmonary metastases
1. Any nodule requires further evaluation with chest CT.
2. Most surgeons advocate chest CT for any patient with recurrent SCCA or with advanced stage III/IV disease since it is more sensitive than CXR.
E. Positron emission testing (PET)
1. Tissues with high metabolic rates (such as tumors) demonstrate increased uptake of radioactive 18-fluorodeoxyglucose (FDG avidity).
2. May be helpful in differentiating post-radiation changes from tumor, and in working up occult nodal disease, pulmonary metastasis, and secondary primaries.
3. Post-treatment response evaluation after chemoradiation (usually 12 weeks after completion).
F. Bone scan evaluates for metastatic lesions in patients with elevated alkaline phosphatase levels, recent fracture, or bone pain.
G. Barium swallow is used to evaluate cervical esophageal involvement if rigid esophagoscopy cannot be performed.
V. HISTOLOGIC DIAGNOSIS
A. Biopsy of the primary tumor can be done in clinic setting with local anesthesia or in OR under general anesthesia depending on anatomic location.
B. Fine-needle aspiration (FNA) of neck masses is used to assess cervical metastasis.
VI. DIRECT LARYNGOSCOPY (DL)
A. Formal evaluation of tumor extension under general anesthesia (“tumor mapping”).
B. Often provides better visualization compared to clinic exam because head and neck musculature is relaxed
C. Rigid esophagoscopy and rigid/flexible bronchoscopy can be performed at the same time to evaluate for synchronous second primary lesions.
VII. ADDITIONAL CONSIDERATIONS
A. Cardiac clearance by cardiology team
B. Nutritional exam
1. Adequate nutrition is imperative for postoperative healing.
2. Patient may require supplemental nutrition.
3. If dysphagia/odynophagia will be problematic, consider nasogastric feeding tube placement.
4. If long-term nutrition will likely be a problem, consider PEG tube.
C. Dental exam: Patients undergoing radiation therapy will need poor dentition extracted prior to treatment in order to avoid caries, abscess formation, and osteoradionecrosis.
D. Pulmonary function tests (PFTs) are required if a patient is being considered for laryngeal conservation surgery (hemilaryngectomy, supracricoid, etc.).
TREATMENT
I. MULTIDISCIPLINARY TEAM MEMBERS
A. Surgical extirpative team
B. Surgical reconstructive team
C. Medical oncologist
D. Radiation oncologist
E. Radiologist
F. Speech therapist
G. Dentist/prosthodontist
H. Nutritionist
I. Physical therapist
J. Social worker
II. ORAL CAVITY AND PHARYNX (EXCLUDING NASOPHARYNX)
A. Single modality treatment (surgery or radiation therapy) for T1/T2 lesions
1. Surgery is favored for oral cavity tumors
a. Better locoregional control and overall survival versus radiation
b. Spares patient radiation side effects (see Complications section)
c. Reserves the use of radiation for recurrence
2. Surgery versus radiation for oropharynx lesions
a. Transoral laser and robotic surgery have evolved as additional options.
b. Chemoradiation of HPV+ advanced oropharynx SCC with excellent survival rates.
c. Patient compliance is imperative when selecting candidates.
d. Early lesions with a high rate of occult nodal metastasis (e.g., oropharynx) should include radiation to neck fields.
e. Disadvantages of XRT: The patient will miss ~2 months of work/activity and tumor recurrence may be difficult to detect in the setting of postradiation changes.
B. Multimodality treatment for T3/T4 lesions
1. Surgery with radiation therapy (usually postoperative)
2. Organ preservation protocols involving chemotherapy (usually cisplatin and 5-FU) and radiation
3. Patients should be educated on available clinical trials
III. LARYNX
A. Glottic SCCA in situ
1. Initially can be treated with vocal cord stripping and close follow-up.
2. Recurrence requires repeat stripping, microlaryngeal excision, radiation, or partial laryngectomy depending on patient history and tumor size.
B. Glottic T1/T2 SCCA
1. Primary radiation with 50 to 70 Gy over 5 to 8 weeks preserves voice quality compared to surgery.
2. Surgery with laser microexcision or partial laryngectomy has an overall cure rate of 80% to 85%.
3. Neck metastases are rare (8%) due to limited lymphatics in glottic region.
C. T3/T4 laryngeal tumors
1. Vertical partial laryngectomy versus total laryngectomy (depending on tumor location and pulmonary status) with postoperative radiation.
2. Organ preservation protocols involving chemotherapy (usually cisplatin and 5-FU) and radiation have equal survival rates versus primary surgery with postoperative radiation.
D. Subglottic SCCA
1. Nodal and cartilage involvement common because presentation is late (presentation often involves airway obstruction).
2. Total laryngectomy with bilateral neck dissections usually required.
3. Postoperative radiation often necessary given late presentation/advanced disease.
4. Stomal recurrence is common (paratracheal node dissection advocated to help prevent stomal recurrence).
E. Speech rehabilitation
1. Esophageal speech: Air released from esophagus vibrates against posterior pharyngeal wall to produce speech.
2. Tracheoesophageal puncture
a. A one-way valve is placed through posterior tracheal wall (~1 cm below stoma opening) into the esophagus.
b. Pulmonary air is diverted through the valve to vibrate against esophagealpharyngeal wall and produce speech.
c. Superior voice quality compared to esophageal speech.
d. Contraindication: Poor patient vision or dexterity; poor patient motivation.
e. Potential complications include leakage, granulation tissue formation, and Candida infections.
3. Artificial larynx (electrolarynx) electronically modulates and amplifies remaining vocal sounds to simulate speech.
A. *Radiation to primary lesion and bilateral necks
B. Concomitant chemotherapy decreases the development of distant metastasis and improves both disease-free and overall survival for advanced disease.
C. Salvage neck dissection required for persistent nodal disease following chemo-therapy and radiation.
V. MANAGEMENT OF THE NECK
A. Selective neck dissection
1. Neck dissection with preservation of one or more lymph node groups.
2. Indication: Used as a staging procedure in a patient without clinical evidence of nodal metastasis (N0 neck) in order to determine the need for postoperative neck radiation.
B. Modified radical neck dissection
1. Removal of all ipsilateral cervical lymph node groups (levels I through V).
2. Preserves at least one of the following vital structures: The internal jugular vein, sternocleidomastoid muscle, or spinal accessory nerve (CN XI).
3. Indication: Treatment of known cervical lymph node metastasis in which the internal jugular vein, SCM, and spinal accessory nerve are not directly involved.
C. Radical neck dissection
1. Removal of all ipsilateral cervical lymph node groups (levels I through V).
2. Removal of all three vital structures: Internal jugular vein, sternocleidomastoid muscle, and spinal accessory nerve (CN XI)
3. Indication: Treatment of advanced cervical disease including multiple, fixed lymph node metastases invading neck structures.
D. Extended neck dissection: Involves additional lymph node groups beyond levels I–V or non-lymphatic structures such as the hypoglossal nerve
VI. RECONSTRUCTION—SEE CHAPTER 16: “PRINCIPLES OF HEAD AND NECK RECONSTRUCTION”
VII. COMPLICATIONS
A. Surgical
1. Bleeding
2. Infection/wound breakdown/potential for carotid artery exposure
3. Scarring
4. Nerve paresis/paralysis (especially marginal mandibular branch of CN VII and spinal accessory nerve)
5. Fistula formation
6. Chronic aspiration
7. Trismus (limited mouth opening)
B. Radiation
1. Xerostomia (dry mouth secondary to salivary gland dysfunction; may be palliated with prosalivatory topical medications)
2. Mucositis: Patient may require PEG or Dobhoff tube for nutrition
3. Pharyngitis
4. Laryngeal and esophageal scarring/stenosis
5. Osteoradionecrosis: Treatment requires debridement, local wound care; ± antibiotics; may eventually require free tissue transfer
6. Dental caries
7. Chronic aspiration
VIII. FOLLOW-UP
A. Routine appointments imperative because HNSCCA has a high rate of locoregional recurrence and of second primary tumor development
1. First year: Every 1 to 2 months
2. Second year: Every 2 to 3 months
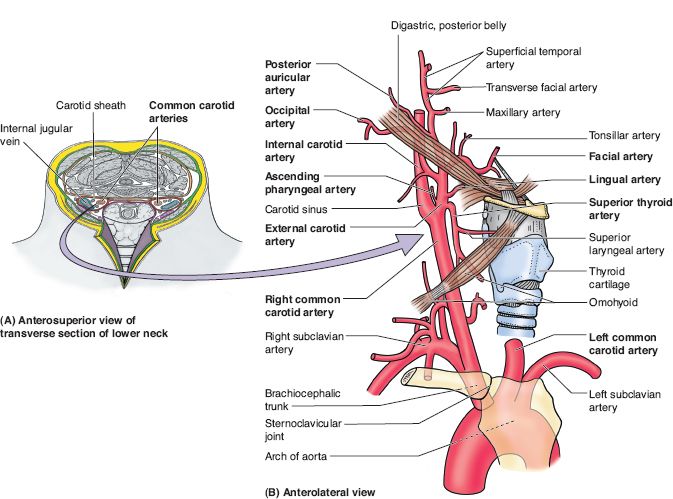
Figure 16-2. Subclavian and carotid arteries and their branches. (From Moore KL, Dalley AF, Agur AM, eds. Clinically Oriented Anatomy. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010.)
3. Third year: Every 3 to 4 months
4. Fourth and fifth year: Every 4 to 6 months
5. Yearly thereafter
B. Yearly chest X-ray to evaluate pulmonary metastasis
C. Radiated patients require yearly TSH because of risk for hypothyroidism
QUESTIONS YOU WILL BE ASKED
1. What are branches of the external carotid artery?
Superior thyroid, ascending pharyngeal, lingual, occipital, facial, posterior auricular, maxillary, and superficial temporal
2. What is the relationship of CN XI to sternocleidomastoid?
The accessory nerve travels approximately 1 cm superior to Erb’s point
3. What is the difference between a modified and radical neck dissection?
Radical neck dissection sacrifices CN XI, IJ vein, and SCM. A modified radical neck dissection spares one or more of these three structures
4. Draw branches of the external carotid artery (Fig. 16-2)
5. Draw the different levels of node dissection
Recommended Readings
Bernier J, Domenge C, Ozsahin M, et al. Postoperative radiation with or without concomitant chemo-therapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945–1952.
Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high risk squamous cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–1944.
Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–2098.
Funk GF, Karnell LH, Robinson RA. Presentation, treatment and outcome of oral cavity cancer: a National Cancer Data Base report. Head Neck. 2002;24:165–180.
Mork J, Lie AK, Glattre E, et al: Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med. 2001;344(15):1125–1131.
O’Malley Jr BW, Weinstein GS, Snyder W, et al. Transoral robotic surgery (TORS) for base of tongue neoplasms. Laryngoscope. 2006;116:1465–1472.
O’Sullivan B, Shah J. New TNM staging criteria for head and neck tumors. Semin Surg Oncol. 2003;21:30–42.
Pfister DG, Ang KK, Brizel DM, et al. National Comprehensive Cancer Network. Head and neck cancers. J Natl Compr Canc Netw. 2011;9(6):596–650. PMID: 21636536.
Robbins KT, Shaha AR, Medina JE, et al. Consensus statement on the classification and terminology of neck dissection. Arch Otolaryngol Head Neck Surg. 2008;134:536–538.
Shah JP. Patterns of cervical lymph node metastasis from squamous carcinomas of the upper aerodigestive tract. Am J Surg. 1990;160:405–409.
The Department of Veterans Affairs Laryngeal Cancer Study Group. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med. 1991;324:1685–1690.
< div class='tao-gold-member'>