Soft Tissue Augmentation: Introduction
|
Method and Technique
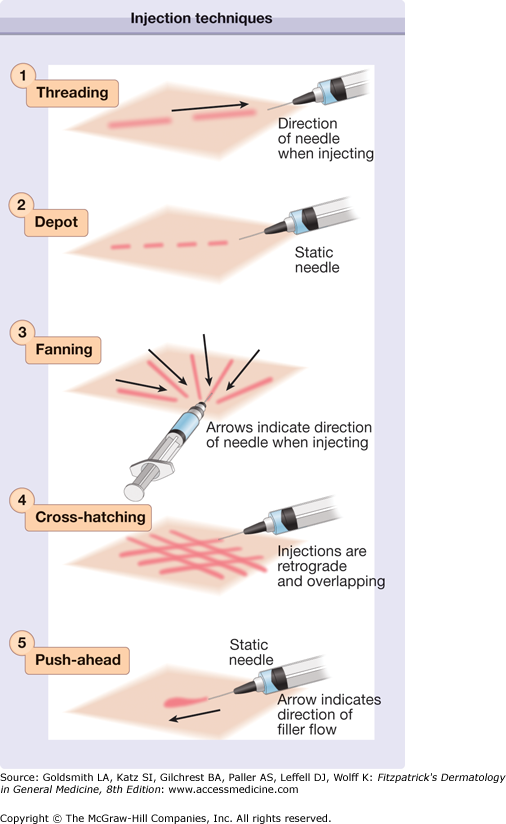
Soft-tissue fillers (Table 254-1) are either injected through a sharp needle or through a blunt cannula. The level of injection into the skin and the chosen length of the needle depend on the type of filler injected, the properties of the filler, the area injected, and the desired result. Threading is a technique in which the needle is inserted into the skin and the filler is deposited in a linear fashion along the track of the needle as it is being withdrawn. Fanning is a type of threading in which, instead of inserting the needle into a new area each time, the needle is just withdrawn so that a new track can be made radially adjacent to the last. In the “push-ahead” technique, an injection is made in an antegrade direction, so that the injectable material flows from the tip of the needle and hydrodissects the tissues as it flows. This technique is often used in areas in which bruising is more likely to occur along the needle track, such as the upper lid and brow. In the depot method of injection, small “pearls” of material are deposited serially, usually along a fold or deep by bone. Cross-hatching is an approach used to diffusely cover an area with the injected material. In this method, linear threads are lined up in succession and a second series of rows is then layered at right angles on top of the first. Figure 254-1 illustrates the different injection techniques. In addition to the methods just described, highly viscous fillers emerging on the US market will most likely be injected in a deep subcutaneous bolus through a blunt cannula or large bore needle. Whatever the technique chosen, care should be taken in highly vascular areas to avoid intravascular injection of filling material. The plunger of the syringe should be pulled back to check for blood flow, and if it is found, the needle should be withdrawn and repositioned.
Filler | Uses | Type | Placement | Complications | Longevity |
|---|---|---|---|---|---|
Hyaluronic Acid | Treatment of medium-to-deep folds, lips, acne scars, periorbital hollows, facial contouring | Non–animal-derived stabilized hyaluronic acid | Mid-to-deep dermis or superficial subutis | Allergic reaction or inflammation, blue discoloration, misplacement, lumps | 6–12 months |
Calcium hydroxylapatite | Treatment of deep folds, nipple reconstruction, nasal reconstruction, jawline, malar augmentation | Calcium hydroxylapatite | Deep dermis to superficial subcutis | Nodules (especially in lips and periorbitally), misplacement with demarcation of product | 6–18 months |
Poly-l-lactic Acid | Treatment of HIV lipoatrophy, nasolabal fold, cheek, and temple hollows | Poly-L-lactic acid | Superficial subcutis | Visible and palpable papules | 2 years or longer |
Autologous fat | Pan-facial filling, especially periorbital area | Autologous fat | Subcutaneous tissue | Bumps, vascular occlusive events with injudicious placement | 5 years or longer |
Silicone | Treatment of scars, HIV lipoatrophy, lips, deep folds | Silicone oil | Deep dermis | Delayed granuloma formation, migration |
Collagens
Hyaluronic Acids
Hyaluronic acid–derived fillers are a good choice for patients who desire a long period of correction or more volume enhancement. They are approved for augmentation of the nasolabial fold, but other common areas of treatment include the labiomental crease, lips, cheeks, and periorbital areas. Patients often feel that the hyaluronic acid fillers are softer and more natural in appearance than the collagens were, but they need to be warned about the risk of increased erythema, edema, and bruising associated with these products.
Hyaluronic acid (HA) is a polysaccharide that is homologous throughout the animal kingdom. Injectable FDA-approved forms include non–animal-derived stabilized hyaluronic acid products (NASHA) made through a bacterial fermentation process and an avian-derived version isolated from cocks’ combs. Brands of injectable hyaluronic acids differ not only in their derivation but also in their concentration of hyaluronic acid per milliliter of product, type of cross-linking or stabilizing agents, viscosity, and particle size. Some hyaluronic acid products exist as biphasic gels containing both crosslinked and uncrosslinked particles and some as monophasic gels containing only crosslinked particles. The biphasic NASHA products are made en bloc initially and then passed through a sieve to create particles ranging in size from 10,000 per mL to 100,000 per mL with a hyaluronic acid concentration of 20 mg/mL. The smaller particle size permits injection through smaller-gauge needles into finer wrinkles and the larger particle size is best for volumetric filling. The small amount of non-cross-linked HA allows for smooth flow with low injection pressures. Monophasic hyaluronic acid gels are produced by varying the amount of high- and low-molecular weight hyaluronic acid producing a hydrogenous gel. The monophasic product is available in two formulations, one containing 24 mg/mL of hyaluronic acid and one containing 30 mg/mL of hyaluronic acid. The crosslinking agent used in both the mono- and biphasic products is BDDE (1,4 butanediol diglycidyl ether). The avian derived hyaluronic acid, contains 6 mg/mL of hyaluronic acid highly cross-linked with divinyl sulfide. All hyaluronic acid products are prepackaged in filled syringes that require no refrigeration.
Due to their high viscosity hyaluronic acid products can cause significant discomfort on injection so most hyaluronic acids are available combined with lidocaine. Topical anesthetic can also be applied before injection. This usually results in adequate anesthesia for periorbital, nasolabial fold, and labiomental crease injections; however, if lips or perioral rhytides are being treated, an ancillary local infiltration of 1% lidocaine or segmental nerve block may be indicated.
Hyaluronic acids should be injected with the patient in a position that elicits the defect or creases to be treated and offers ease of injection to the operator. This is best accomplished by reclining the patient to a 45° angle. All the hyaluronic acid products come with a needle in the gauge of choice for ease of injection and preservation of the physical properties of the gel. Due to their viscoelastic properties, however, hyaluronic acids can often be injected through needles tailored to the area or level of injection. For instance, a 32-gauge needle allows for more precise injection of vertical lip rhytides and a 1½ inch needle may facilitate injection into distal sites. Hyaluronic acids are injected in most areas via threading, depot, or fanning. They are particularly amenable to cross-hatching in areas in which a greater density of augmentation is desired. In particular, when hyaluronic acid is injected into the nasolabial fold it is important to span the width of the fold with a serial threading technique and then crosshatch at right angles. This gives structural integrity to the fold and prevents the undesirable result of merely moving the fold medially. In addition, it is often necessary to “suspend” the fold by augmenting the cheek. This is usually done with a deep bolus injection of a high density HA. The labiomental crease can be injected similarly to the nasolabial fold, again blending the product so as not to move the fold medially. Lip injections are made using a depot or threading technique, either along the vermillion border, in the body of the lip, or in a combination of both. Perhaps one of the most exciting off-label applications of hyaluronic acids is the correction of tear trough deformity or suborbital hollows. Injections are made either under the orbicularis oculi muscle near the orbital bone or subdermally above the muscle. Intramuscular injections are to be avoided since the movement in this area increases the risk of lumping of the product. A depot method of injection is preferred in which very small amounts of hyaluronic acid are deposited on each pass, but threading can also be helpful to ensure even blending into neighboring areas. The push-ahead technique is preferred for the sub-brow area. After injection the periorbital area should be vigorously massaged to disperse the product and minimize aggregation of gel particles. Although periorbital injection of hyaluronic acid results in high levels of patient satisfaction, it is an advanced injection method best left to those with the greatest injecting experience.
The application of ice immediately after the procedure and periodically throughout the day is recommended after the injection of hyaluronic acid. Patients are instructed to avoid manipulation of the treated area and extremes of temperature for the first 48 hours after injection. If the lips were treated in a patient with a history of cold sores, then an antiviral medication should be administered prophylactically on the day of the procedure. Patients are instructed to return to the office if they experience any problems such as redness, purulence, or nodule formation.
Most complications result from improper application of the product. Placement of this clear gel too superficially results in a blue discoloration. Placement of too large an aliquot can result in a noninflammatory bump. Most ill-placed hyaluronic acid can be released by incising with a 20-gauge needle and expressing the material. Hyaluronic acid products also contain small amounts of impurities that can cause hypersensitivity reactions. Patients can react to sterile bacterial proteins or avian proteins by forming sterile abscesses or granulomatous inflammatory nodules. It has been shown that these nodules, which appear clinically to be granulomas, may in fact be small foci of infection. Treatment with an antistaphylococcal antibiotic is the initial treatment of choice. If no resolution occurs, dilute intralesional corticosteroids can be administered, or hyaluronidase can be injected to cause rapid dissolution of the product.1
In general, the longevity of augmentation is greater for the more highly cross-linked or stabilized forms and increases with increasing viscosity and particle size. Hyaluronic acid–based products are broken down via isovolemic degradation; they maintain a constant volume throughout their degradation because of their ability to bind water. This property makes possible a very long-lasting product.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree