Olfaction and taste promote satisfaction and protection in daily life. The astute facial plastic surgeon recognizes the importance of a baseline smell test to document the patients’ olfactory status before surgery. After surgery, the surgeon must be alert to the possible mechanisms of hyposmia and anosmia and the pertinent treatment strategies. The surgeon must also understand the importance of counseling the patient and family regarding the cause of the dysfunction and the proper treatments. This article updates the facial plastic surgeon on the importance of smell and taste and associated disorders with a current review of the literature.
The chemosenses of smell and taste contribute to quality of life and environmental appreciation. Not only do these senses guard against toxic and dangerous stimuli, but the ability to smell and taste contributes to the finer qualities of life, including participating in wine tasting, detecting a fresh-brewed steaming cup of coffee, or baking Christmas cookies. Nasal surgery can alter a patient’s olfaction and some patients have unrecognized olfactory loss before surgery. A recent study from Turkey found that practitioners who were educated about olfactory loss were more likely to rate smell loss as important and be more confident in managing smell loss. We advocate that a basic smell test and record of a patient’s olfaction before surgery is key to avoiding both patient injury and legal misfortune. A working knowledge and awareness of the many factors that contribute to a patient’s sense of smell (and taste) is fundamental.
- •
Olfactory testing is crucial for accurately diagnosing chemosensory problems and before nasal surgery as a medical-legal safeguard for a medical practice. There are several easy-to-use smell and taste tests.
- •
Olfaction is a complex process involving both orthonasal and retronasal pathways for olfactory stimulation.
- •
The astute surgeon may identify nasal and systemic disease factors that may play a role in postoperative olfactory loss.
- •
The presence of functioning senses of taste and smell is a major boost to quality of life and safety.
Surgeons must pay attention to surgical risk factors, surgical techniques, and existing preoperative disease because these can lead to olfactory disturbances and permanent smell loss after surgery.
Anatomy
Olfaction
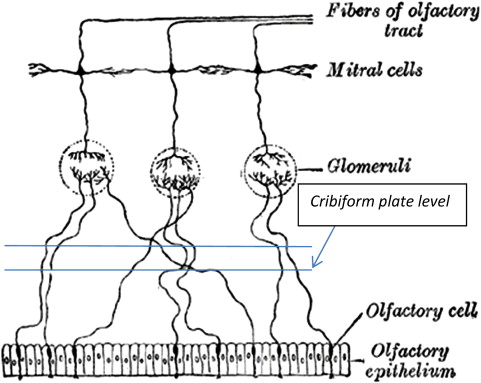
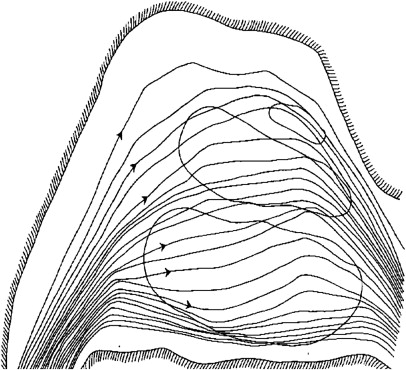
The organs of olfaction are unique within the human body because they contain neuroepithelium that regenerates. However, these delicate nerve fibers may also be damaged and undergo permanent loss. Grossly, the nasal passages contain the structures of olfaction, which include the upper nasal septum, and middle and superior turbinates. These structures facilitate airflow and contain the primary olfactory neurons. Olfactory molecules dissolve in mucous overlying these neurons and then interact with the neurons, producing an action potential. The olfactory bulb, which is positioned at the anterior cranial fossa, serves as the initial transfer station in the olfactory pathway ( Fig. 1 ). The primary olfactory neurons synapse with secondary neurons, which constitutes aggregates referred to as glomeruli. The olfactory cleft lies in a protected location approximately 7 cm from the nasal sill ( Fig. 2 ). Olfactory bulb comes from the Latin words olfactus , which means sense of smell, and bulbus , which means swollen root. This region is where the olfactory nerves (cranial nerve I) terminate and the olfactory tracts arise.
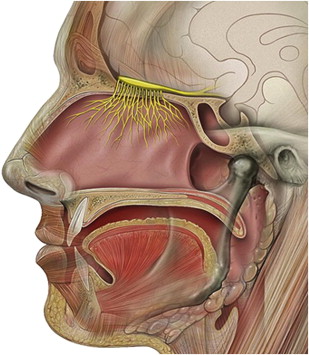
Cranial nerve I mediates the olfactory response with additional input from cranial nerves V, IX, and X. The trigeminal nerve relays pungent or irritating odors such as carbon dioxide, which is known to be a pure trigeminal stimulant. Cranial nerves IX and X assist in retronasal olfaction, which is further discussed in this article. The human olfactory epithelium covers an area of roughly 1 cm 2 on each side. Humans have approximately 6 million olfactory neurons.
Vomeronasal Organ
In more than 80% of adults, there is a pit or cleft located in the anteroinferior nasal septum. This pit contains neuroepithelium-like tissue that has no neural connection to the brain. Explanations for its existence include that is a vestigial olfactory organ (which functions in many other animals), or that it has a neuroendocrine function. The data to date suggest that it has no functional significance.
Microhistology
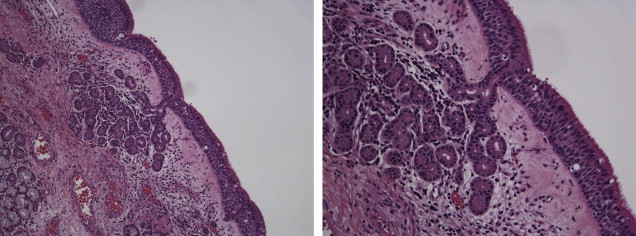
The olfactory epithelium is primarily composed of pseudostratified columnar-type epithelium, is situated with a vascular lamina propria, and lacks a submucosa ( Fig. 3 ). The cells that facilitate olfaction are divided into 4 main cell types: ciliated olfactory receptors, microvillar cells, sustentacular cells, and basal cells. In the olfactory mucosa, the axons from the bipolar olfactory neurons coalesce into bundles to form cranial nerve I ( Fig. 4 ). This nerve traverses upward through the cribriform plate and skull base to the olfactory bulb. A complex olfactory map brings signals to higher processing centers, which is thought to be partly responsible for olfactory coding.
Clinical Olfaction
Definitions
Loss of the sense of smell is subdivided into categories dependent on the degree of loss, dysfunction, or altered perception. It is important to correctly identify and document the type of loss or dysfunction because each has distinct prognosis and treatments.
- •
Normosmia is used to describe the perception of an intact sense of smell
- •
Hyposmia describes a decreased ability to perceive smell
- •
Anosmia describes the absence of useful smelling ability
- •
Parosmia is the distorted perception of odor following a stimulus
- •
Phantosmia is perception of odor in the absence of an odorant stimulus.
Taste
Definitions
Most patient-described losses or disturbances of taste are olfactory losses. True solitary taste loss is less common.
- •
Hypogeusia is diminished sense of taste
- •
Ageusia is absence of taste
- •
Dysgeusia is altered or distorted taste sensation.
Taste (Gustatory) Anatomy
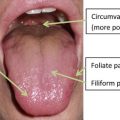
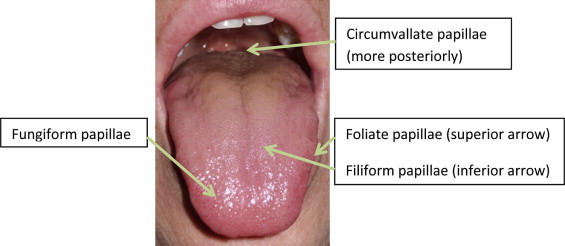
The oral cavity contains taste receptors (taste buds within papilla) largely on the tongue but also on the palate, pharynx, and epiglottis. Grossly, the tongue is composed of multiple taste papillae geographically distributed. Fungiform, circumvallate, and foliate papillae contain taste buds that facilitate gustatory sensation ( Fig. 5 ). The filiform papillas do not contribute to taste but are readily distributed in the tongue. The chorda tympani (cranial nerve VII) mediates taste from the anterior two-thirds of the tongue. The glossopharyngeal nerve (cranial nerve IX) mediates the posterior one-third of the tongue and circumvallate papilla. The vagus facilitates sensation from the posterior pharynx and laryngeal epiglottis. Taste neurons also regenerate, and are replaced approximately every 10 days.
Physiology of smell
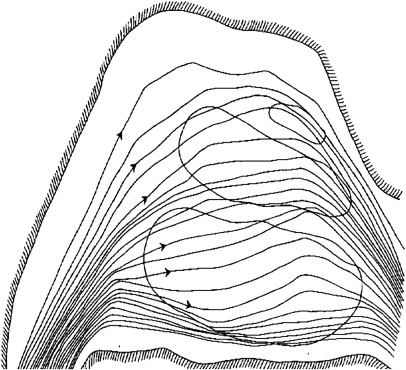
Nasal airflow plays an integral part of smell detection. Although olfactory particles can contact the olfactory cleft by diffusion, airflow is generally a prerequisite of olfaction. As airflow distributes itself in the nasal cavity, about 15% flows to the olfactory cleft ( Fig. 6 ). Smell is typically thought of as being mediated through nasal inhalation with orthonasal flow. Another contributor to olfaction and food flavor perception is retronasal olfaction, which occurs during ingestion of substances with airflow of odorant molecules generated by exhalation, or mouth and pharynx contraction. This phenomenon is important clinically because a group of patients display adequate retronasal olfactory function with modest or no orthonasal function remaining, and yet these patients recognize smell. The differences between orthonasal and retronasal perception of odor are at least partly caused by absorption of odors to the olfactory epithelium, which seems to differ in relation to the direction of the airflow across the olfactory epithelium. This concept favors a duality of smell.
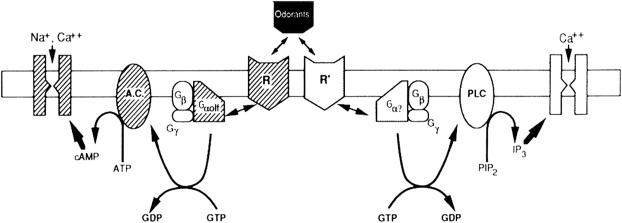
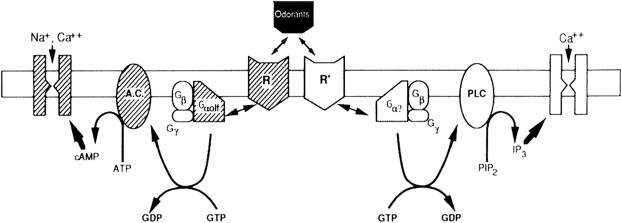
Inherently, key to this process is that odorant molecules must dissolve in or pass through the mucous overlying the olfactory epithelium to be detected. Once the odorants bind to the receptor neurons, a complex enzyme-mediated pathway ensues ( Fig. 7 ). This chemical and electrical pathway transmits neuronal information to higher centers involving both the frontal lobes and temporal lobes. These pathways are complex and interweaving, allowing humans to associate memories with a particular smell and event. For example, the smell of a fir tree makes many people recall Christmas time.
Physiology of smell
Nasal airflow plays an integral part of smell detection. Although olfactory particles can contact the olfactory cleft by diffusion, airflow is generally a prerequisite of olfaction. As airflow distributes itself in the nasal cavity, about 15% flows to the olfactory cleft ( Fig. 6 ). Smell is typically thought of as being mediated through nasal inhalation with orthonasal flow. Another contributor to olfaction and food flavor perception is retronasal olfaction, which occurs during ingestion of substances with airflow of odorant molecules generated by exhalation, or mouth and pharynx contraction. This phenomenon is important clinically because a group of patients display adequate retronasal olfactory function with modest or no orthonasal function remaining, and yet these patients recognize smell. The differences between orthonasal and retronasal perception of odor are at least partly caused by absorption of odors to the olfactory epithelium, which seems to differ in relation to the direction of the airflow across the olfactory epithelium. This concept favors a duality of smell.
Inherently, key to this process is that odorant molecules must dissolve in or pass through the mucous overlying the olfactory epithelium to be detected. Once the odorants bind to the receptor neurons, a complex enzyme-mediated pathway ensues ( Fig. 7 ). This chemical and electrical pathway transmits neuronal information to higher centers involving both the frontal lobes and temporal lobes. These pathways are complex and interweaving, allowing humans to associate memories with a particular smell and event. For example, the smell of a fir tree makes many people recall Christmas time.
Physiology of taste
Traditionally, taste is subdivided into 5 major gustatory classifications: salty, sweet, bitter, sour, and umami. In the taste buds, the chemical molecules are transformed into electrical signals that travel to the nucleus solitarius (medulla), to the ventricular posterior medial nucleus in the thalamus and to the parietal lobe, and give the perception of gustation.
Another integral component of gustation is the pleasure related to the oral sensation and tastes of the food ingested. Genetics play a role in what is interpreted as pleasurable, and obesity has been linked with the feel of foods. A group of receptors, T1R1-3s, have been found to detect sweet tastes. Conversely, the taste of certain foods protect from toxins and other harmful substances, and an even larger collection of T2R receptors facilitates bitter taste sensation. A sensation dependent on the lipid content of the food, described as fatty taste, may increase the pleasantness derived from tasting food. This sensation has been studied in rats and miceand has been linked to circumvallate papillae function.
Diagnosis and workup of olfactory complaints
Clinical History of Olfactory or Taste Loss
A discussion with the patient while obtaining a history is useful for identifying the onset, symptoms, and duration of smell and taste changes. The patient may not disclose an olfactory disturbance unless directly asked. Associated clinical clues such as preceding trauma or viral infection are helpful in determining the cause of the perceived loss. According to rough projections, at least 1% of patients have an unrecognized loss. One study found that up to 16% of the general population has an olfactory disturbance. Patients may also complain of taste disturbance instead of an olfactory loss, which should increase the surgeon’s suspicion of a probable smell disturbance because the 2 senses are intimately linked. A brief discussion of medications for association with dysgeusia is also warranted.
Physical Examination for Olfaction
The facial plastic surgeon will assess the patient’s nose for aesthetics and functional deformities, dimensions, and collapsibility of skin, cartilage, and nasal valve. A brief examination of the nasal cavity on anterior rhinoscopy may reveal gross disease such as septal perforation, polyps, systemic disease, and epistaxis, tumors, or allergic edematous nasal mucosa. Documentation of the physical examination is vital before surgery. Neurologic status and cranial nerve function should be assessed. If the surgeon performs nasal endoscopy, a view of the olfactory cleft and assessment of blockage may predict olfactory dysfunction. Tumors or polyps may also be ruled out on highly sensitive nasal endoscopy. A comprehensive description of appropriate workup is given in Fig. 8 .
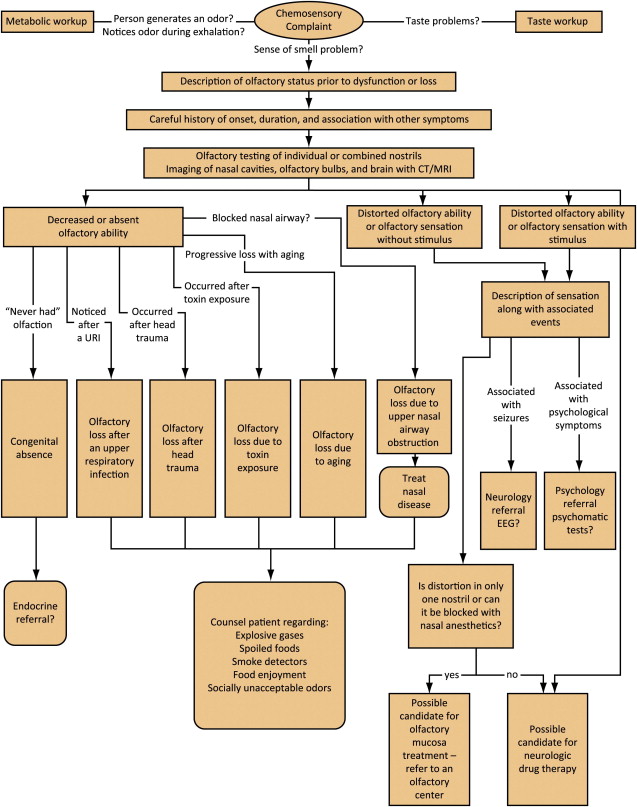
Chemosensory testing
Olfactory Testing
Two classes of testing are available: electrophysical and psychophysical tests; however, psychophysical tests are more useful in the interoffice setting, more widely used, simple, and easy for the plastic rhinoplasty surgeon to use. Electrophysical tests are generally reserved for research studies. Testing olfaction documents not only the extent of the olfactory loss but also allows for comparisons during clinical follow-up and assessment for improvement over time (duration of deficit). Olfactory testing generally measures either threshold of smell or identification of various smells. A wide variety of tests are available in the olfactory work-up. Surgeons working on or around the nose should be familiar with the benefits and disadvantages of these tests ( Table 1 ).
| Name | Type | Advantages | Disadvantages |
|---|---|---|---|
| Alcohol pad Threshold tests (pyridine) | Threshold Threshold | Quick, simple, inexpensive, can test unilateral function Simple | Less rigorous Low test-retest reliability, time intensive |
| Smell identification tests | Identification, suprathreshold | Compares normative data, fun, portable, test for malingering | Requires grading |
| Sniffin’ sticks | Threshold and identification | Reusable, rapid administration | Availability, expensive |
| T & T olfactometer | Threshold | Standardized in Japan, graph data | More labor intensive |
Alcohol pad test
Perhaps the simplest tests include the use of an isopropyl alcohol wipe pad to detect degrees of hyposmia or anosmia. The patient with eyes closed has an alcohol pad slowly brought from a distance to close to the nose. The patient states when the odor is first perceived, and this distance from the examiner and alcohol pad correlates with the degree of olfactory loss (see Table 1 ). The test has been able to differentiate hyposmic and anosmic patients and can be modified for unilateral testing.
Odorant tests
Threshold tests with different odorants, most commonly pyridine and n -butyl alcohol (1-butanol), and phenylethyl alcohol rose smell (less trigeminal stimulation than the others), test the patient’s detection threshold at different concentrations ranging from the least concentrated to the most concentrated odorant. The patient may also be presented with either a bottle containing the odorant or a placebo and be asked to choose the one with the odorant. Drawbacks to this test are low test-retest reliability, the time required for testing, and the need for a tester.
Smell identification tests are used worldwide and are available with culture-specific data. Microencapsulated odorants make these tests easy for self-administration ( Fig. 9 ). The patient is presented with suprathreshold levels of odorants and asked to correctly identify the various odorants. Normative age-related and sex-related data via percentages are accessible. Countries such as Brazil and Australia have developed their own normative data.

In other parts of the world, olfactory testing is also being performed, sometimes with one of the tests noted previously and sometimes with locally designed tests. In Japan, the standard test is the T&T olfactometer, which is a rack containing 8 concentrations of 5 different odorants. From this test, both detection and recognition thresholds can be determined, and they are charted on a graph similar to an audiogram.
In Germany, an odorant threshold and identification forced-choice test that uses odorant-impregnated felt-tip pens has been developed. It can be reused and has short test administration times ( Fig. 10 ).
Computed tomography scans
If the history, physical examination, or olfactory tests suggest polypoid or obstructive disease or malignant potential, then sinonasal imaging is completed. Computed tomography (CT) scans of the paranasal region, principally in the coronal plane, are beneficial in assessing any anatomic abnormalities, tumors, malignancy, or obstructive disorder. Bony definition is particularly well defined on CT scan. Magnetic resonance imaging (MRI) gives an inadequate portrayal of bony detail, but may visualize the olfactory sulci and brain structure.
Clinical evaluation of taste
History
Patients presenting with a complaint of taste disturbance should be asked about any associated disorder of smell; any preexisting medical conditions and their treatment, such as ear infection, ear surgery, Bell palsy, significant head injury, recent upper respiratory tract illness, and various dental procedures or prostheses. A detailed medication history also should be obtained.
In addition to a physical examination and testing of taste and smell, special attention should be paid to the oral cavity for evidence of infection, inflammation, degeneration, and masses, as well as atrophy and dryness of tongue, gums, dentition, and surrounding mucous membranes. Specific investigations are ordered to identify suspected causative considerations suggested by the clinical features. If no local cause is suggested, patients with taste abnormalities, particularly unilateral, should undergo audiologic evaluation and imaging studies to include the middle ear.
The sense of taste can be tested with readily available stimuli, such as aqueous solutions of sugar, sodium chloride, acetic acid, and quinine, or with electrical stimulation of the tongue (electrogustometry). A cotton applicator is used to rub the aqueous solution gently onto the lateral quadrant of the protruded tongue. The patient should not withdraw the protruded tongue, close the mouth, or talk, but should identify the perceived taste by pointing to cards printed with the words sweet, salt, sour, and bitter. The mouth is then rinsed with water between tests. Electrogustometry is the evaluation of taste by applying graded electrical currents to the tongue to produce a sensation described as sour or metallic. In normal subjects, the 2 sides of the tongue have similar thresholds for electrical stimulation, rarely differing by more than 25%. The technique has the advantages of simplicity, speed, and ease of quantification, and is capable of providing a reliable objective recording of the gustatory detection threshold.
Common Causes of Olfactory Loss
Disorders of olfaction are common worldwide and may involve as many as 2 to 4 million people in the United States. As previously defined, wide variations occur in perceptions of smell, and testing is available to assist in delineation of the degree of loss. Olfactory disturbances can be better separated into those with a conductive component (anatomic blockage of the olfactory cleft or surrounding structures) and a sensorineural component (a nerve loss or damage to receptor or higher cortical processing). This distinction is important for distinguishing those who have chance of improvement and treatment. Generally, conductive losses are treatable.
Conductive Losses (Nasal Obstructions)
Nasal inflammatory disease
Nasal inflammatory disease encompasses a variety of nasal disorders from chronic rhinosinusitis to polyps or allergic edema. This group of disorders is thought to primarily alter nasal airflow to the olfactory cleft; however, there is evidence that a sensorineural component and changes in the neural olfactory epithelium on histologic examination can occur ( Fig. 11 ).