It is important to understand the histology and physiology of skin for the prediction and optimization of wound healing. Optimal postoperative wound healing to minimize scarring entails minimizing local, systemic, and environmental factors that lead to poor wound healing. Keeping the wound clean and moist, minimizing trauma, and infection are the local wound tenets. Systemic tenets include minimizing medications that inhibit processes of wound healing, maintaining adequate nutrition, pain palliation, UV protection, and smoking cessation. This article presents the dynamic process of wound healing and the basic tenets to minimize scarring.
- 1.
Skin is composed of several layers that are essential to its function and response to injury: the epidermis, dermis, and hypodermis. Healing is a dynamic progression encompassing hemostasis, inflammation, proliferation, and remodeling
- 2.
Pilosebaceous units are the source of all epithelial stem cells essential for reepithelialization and wound healing
- 3.
Multiple extrinsic and intrinsic factors affect healing, specifically the effect of immune system modulation (medications and diseased states)
- 4.
It is most optimal to wait at least 4–6 weeks after smoking cessation for elective surgical interventions
- 5.
Keloids and hypertrophic scarring are a result of overabundant collagen production, and decrease collagen breakdown. Keloids are difficult to treat, due to their recurrent nature. It is important to identify individuals prone to keloid formation for surgical planning purposes
Self-Test Questions
The following questions are intended for the reader to self-test. The answers, with full background, are covered within this article. The correct answers are provided at the conclusion of the article.
- 1.
A 19-year-old woman is on isotretinoin (Accutane) for acne and has a facial acne scar that she wishes to be dermabraded. What do you counsel the patient about?
- a.
She needs to be off the medication for 1 year to limit the risk of scarring
- b.
She should continue the medication because the extra vitamin A will improve her healing
- c.
You cannot resurface her acne scar because of the long-lasting effects of this medication
- d.
Encourage 2 g of vitamin C daily for 2 weeks before the procedure
- a.
- 2.
A 73-year-old insulin-dependent diabetic man with a serum glucose level of 300 mmol/L comes to your office for a rhytidectomy. How do you optimize your results?
- a.
Refer to endocrinologist for tight diabetic control before surgery
- b.
Decline the surgery because the risk of failure is increased
- c.
Start the patient on vitamin E supplementation 2000 IE daily 2 weeks before surgery
- d.
Double his insulin dose on the morning of his surgery
- a.
- 3.
A 30-year-old man has a partial-thickness 4 × 4-cm abrasion on his right cheek. What should be the best treatment?
- a.
Place a split-thickness skin graft
- b.
Place a full-thickness skin graft
- c.
Keep the wound bed moist with a moisture retentive ointment
- d.
Keep the wound dry to maximize reepithelialization.
- a.
- 4.
A 50-year-old man sees you about a large wide scar on his neck. On inquiring, the patient states he had a lymph node removed last year and this scar has grown bigger than the cyst was. What do you counsel him about?
- a.
That he most likely has a hypertrophic scar and that the likelihood is that this will not happen on further surgical procedures
- b.
That this is a keloid and that with vitamin E ointments it should resolve
- c.
That this is a hypertrophic scar that will completely go away with simple excision
- d.
That this is a keloid and that multiple procedures along with steroid injections may be required to excise it, but still there is no guarantee it will be removed completely.
- a.
Skin histology
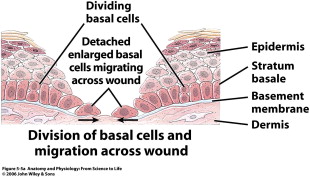
Keratinocytes make up 95% of the epidermis, and the stratum basale is the source of all replicating keratinocytes. It is these keratinocytes in the basal layer that are primarily responsible for the epidermal response in wound healing. As keratinocytes replicate they push older cells toward the surface, and these cells progressively lose their nucleus and take on a more flattened ovoid shape. This stratified squamous keratinized epithelium undergoes constant turnover, essentially regenerating fully every 48 days. The stratum basale sends down finger-like projections that interdigitate with similar structures reaching up from the dermis. This process forms the rete ridges that are often seen in cross section. Freshly healing wounds as well as skin grafts lack these rete ridges initially, which makes them susceptible to shear trauma early on.
The epidermis also contains essential appendages including hair follicles with associated sebaceous glands (pilosebaceous unit), eccrine sweat glands, and apocrine glands. The pilosebaceous unit, as well as rete ridges, contains epithelial stem cells that are able to regenerate and differentiate into basal keratinocytes and are essential to the reepithelialization process ( Fig. 2 ). These stem cells are critical, as they are relatively undifferentiated, have a large proliferation potential, and have a high capacity for self-renewal. Healing difficulties are seen when these stem cells are destroyed by insults such as burns and even iatrogenic sources such as dermabrasion. When dermabrasion or resurfacing is taken too deep it can destroy the apocrine glands and pilosebaceous unit, leading to improper healing and eventual scarring. Retinoid treatments such as isotretinoin (Accutane) cause atrophy of the sebaceous glands, which is the source of their effectiveness to treat acne. Isotretinoin leads to obvious problems with wound healing, directly reducing the cells needed for reepithelialization. Most surgeons would agree that patients should be off isotretinoin for at least 1 year prior to any surgical resurfacing procedure.
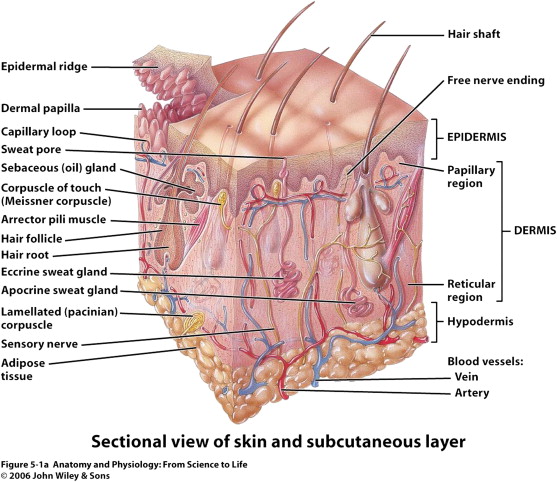
The dermis is the next layer down, which receives the major blood supply for the skin and contains most of the dermal appendages of the skin including the apocrine glands, eccrine glands, and hair follicles. This layer itself is separated into a superficial or papillary dermis and the deeper reticular dermis. As a general rule, any damage that extends into this deeper reticular layer will invariably cause scarring and may require repair with full-thickness grafts or flaps to assure proper healing.
In general there are 4 overlapping phases of wound healing ( Fig. 3 ):
- 1.
Hemostasis
- 2.
Inflammation
- 3.
Proliferation
- 4.
Maturation/remodeling.
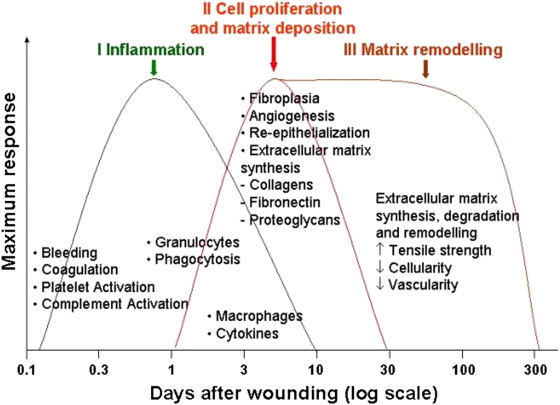
Although these phases are separated for simplicity reasons they in fact overlap a great deal, and even different areas of wounds can be in different phases of healing. Any interruption in the natural cascade of healing can disrupt subsequent phases and can potentially result in abnormal healing, chronic wounds, and eventual scarring.
Hemostasis
Hemostasis is the initial phase that occurs within seconds to minutes after the initial insult. Initially hemorrhage into the wound exposes platelets to the thrombogenic subendothelium. Platelets are integral to this phase and the entire healing pathway, as they not only serve to provide initial hemostasis but also release multiple cytokines, hormones, and chemokines to set off the remaining phases of healing. Vasoactive substances such as catecholamines and serotonin act via specialized receptors on the endothelium to cause vasoconstriction of the surrounding blood vessels. Smaller vessels are signaled to vasodilate to allow the influx of leukocytes, red blood cells, and plasma proteins. Platelets interact with the GpIIb-IIIa receptor on the collagen of the damaged subendothelium to become activated and form an initial clot. Activated platelets release their granules and ignite the extrinsic and intrinsic coagulation cascades. Fibrin polymerization helps form a mature clot and serves as scaffolding for the infiltrating cells (leukocytes, keratinocytes, and fibroblasts) that are key to subsequent phases of healing. Platelets release a myriad of chemokines and cytokines to attract these inflammatory cells to the area. Within minutes, there is an influx of inflammatory cells (predominantly neutrophils and macrophages), leading to the next phase of healing.
Inflammation
The inflammatory phase is heralded by the influx of neutrophils, macrophages, and lymphocytes to the site of injury ( Fig. 4 ). Neutrophils are the first leukocytes on site, arriving en masse within the first 24 hours. Neutrophils are soon followed by macrophages, which are attracted by the by-products of neutrophil apoptosis. Phagocytic cells such as macrophages and other lymphocytes appear in the wound to begin to clear debris and bacteria from the wound. These macrophages infiltrate at approximately 48 hours post injury and stay until the conclusion of the inflammatory phase. Macrophages have long been thought to be the key cell to the wound-healing process, and they seem to orchestrate the most important phases of healing. Although they are integral to proper healing, recent research has also looked at their role in improper healing and scarring. Studies of macrophage function have revealed that these key cells are intricate in reepithelialization, granulation tissue formation, angiogenesis, wound cytokine production, and wound contracture. Inflammation is a necessary step of the healing process, and inhibition of this key phase (via anti-inflammatory medications) can result in improper healing. This phase of healing is important to combat infection. If disrupted or prolonged (ie, longer than 3 weeks), this inflammation can lead to a chronic wound, impaired healing, and eventually more scarring. Important factors that can abnormally lengthen this phase of healing include high bacterial load (greater than 10 5 microorganisms per gram of tissue), repeated trauma, and persistent foreign material in the wound. Once the wound has been debrided, the repair or proliferative phase begins.
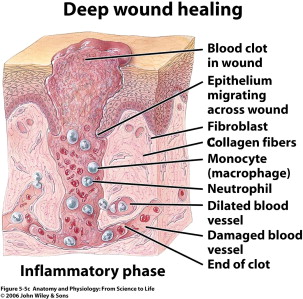
Proliferation
The proliferative (repair) phase begins early on in the form of reepithelialization ( Fig. 5 ). The repair phase also involves capillary budding and extracellular matrix production to fill in the defects left behind from debridement of the wound. Epithelialization is marked by the proliferation and influx of keratinocytes near the leading edge of the wound. As discussed previously, stem cells within the bulbs of the hair follicles and apocrine glands begin to differentiate into keratinocytes and repopulate the stratum basale, and also begin to migrate over the edge of the wound. Once they encounter the mesenchyme of the extracellular matrix (ECM), they attach near the inner wound edge and begin to lay down a new basement membrane. Following this, another row of keratinocytes migrates over the newly laid epithelial cells to fill in the defect. These cells migrate and digest the ECM using proteases until they are in physical contact and they stop migrating, signaled by contact inhibition from neighboring keratinocytes. This reepithelialization protects the wound from infection and desiccation. During this process a layer of uninfected exudates lies over the wound, which provides an important moisture layer and contains growth factors essential to healing. Any improper wound dressings that destroy this healthy layer will result in delayed healing. Underneath this reepithelialization process the ECM continues to be laid down. Wounds healing by secondary intention fill in with granulation tissue. Under the influence of vascular endothelial growth factor (VEGF), angiogenesis begins as the vessels begin to bud from the blood vessels surrounding the wound. Granulation tissue consists of these fibroblasts, new budding vessels, and immature collagen (collagen type III). Some fibroblasts will also begin to differentiate in this phase into myofibroblasts that have contractile function to bring gaping wound edges together.
Angiogenesis/Maturation
Angiogenesis follows a typical pattern of sprouting, looping, and pruning signaled by a complex gradient of cytokines. These small and delicate sprouting vessels repopulate the dermis, and any trauma to this area will lead to destruction of these vessels and delayed healing. Shearing of these new vessels is often a problem in skin grafting, whereby the graft is solely supplied by initial imbibition for 24 hours followed by growth of these vessels into the tissue. It is the role of the bolster placed over these delicate graft sites to prevent hematoma that would limit diffusion of nutrients, but more importantly to prevent shearing of these delicate immature vessels. Bolsters are usually left on for 5 to 7 days to allow these vessels to become more robust and mature, and to resist these shearing forces. Angiogenesis is also a key process affected by primary versus secondary closures. Angiogenesis is greatly accelerated by primary closure, due to the proximity of the budding vessels. In healing through secondary intention, this process takes place through formation of the aforementioned granulation tissue induced by hypoxia, elevated lactate, and various growth factors. Healing by secondary intention involves epithelialization over this granulation and then extensive remodeling. Any medications that interfere with new blood vessel formation (ie, the antiangiogenic drug bevacizumab [Avastin]) can be detrimental to wound healing.
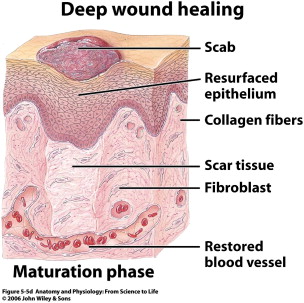
Remodeling
The remodeling phase begins as the provisional ECM and type III collagen is replaced with type I collagen and the remaining cell types of the previous phases undergo apoptosis ( Fig. 6 ). With the laying down of type I collagen, the tensile strength of the wound dramatically increases. Granulation tissue begins to involute and excess blood vessels retract. This phase lasts the longest and results in the final appearance of the wound following healing. A successful remodeling phase involves a delicate balance requiring synthesis more than lysis. Synthesis is greatly energy dependent, and any depletion of nutrients will push the balance toward lysis and affect the healing process. Excess fibrosis at this stage results in hypertrophic scarring (with the scar limited to the wound area) or keloid formation (with the scar extending beyond wound edge). The difficulty in treating both of these entities has targeted many research dollars on the prevention of scarring, and is discussed by Douglas Sidle, in detail in an article elsewhere in this issue.
Primary and Secondary Healing
It is important to discuss in brief the differences between primary and secondary healing. Primary healing is that which is seen in surgical wounds that involve uncomplicated healing of noninfected, well-approximated wounds ( Fig. 7 ). These wounds follow the 4 phases of healing without abruption. Any disruptions in healing such as infection, dehiscence, hypoxia, or immune dysfunction will lead to a compromised wound that enters a stage of secondary healing. If no intervention is undertaken, these wounds may become chronic wounds. Chronic wounds are out of the purview of this article but they warrant a comprehensive approach for treatment, which obviates the need to monitor surgical wounds. One needs to monitor for the two most common complications, bleeding and infection, using the old tenets of “dolor, rubor, tumor, and calor” (pain, redness, growth, and heat). Healing by secondary intention, as discussed previously, involves creation of granulation tissue and epithelialization over this healthy granulation tissue. Because angiogenesis and epithelialization takes longer in this setting, they are more prone to infection and poor healing. With proper care, healing by secondary intention may result in acceptable cosmesis on concave surfaces of the face.
Collagen in Wound Healing
Collagen is the single most abundant protein in mammals, and accounts for approximately 30% of our body’s total protein content. Collagen and its triple helical structure have been studied for decades, and its structure was first described in the 1930s. Collagen is made from 3 alpha strands of left-handed helices that coil together to form the right-handed helix of a collagen fibril. These fibrils cross-link together via hydrogen bonds to form fibers. The molecular structure of collagen, as for all proteins, is essentially amino acids. The regular arrangement of collagen amino acids (Gly-Pro-X) provides for a great amount of noncovalent bonding. The most important step in the synthesis of collagen is the vitamin C–dependent hydroxylation of the proline. It is this step that provides the tensile strength of collagen and, consequently, wound healing. The depletion of vitamin C in sailors led to scurvy and, ultimately, to poor wound healing and loss of dentition in this population. This major step is also inhibited by systemic steroids, thus resulting in poor wound strength and delayed healing. Hyperbaric oxygen also acts at this step, catalyzing the hydroxylation and improving wound strength as well as accelerating healing.
Wound Strength
Wound strength follows a typical curve in an ideal situation ( Fig. 8 ). As one can see, the wound strength begins to plateau around 4 to 5 weeks, reaching around 60% of its original strength. Once healed, it reaches its maximum strength (only 80% of its original) at approximately 1 year. This curve has important implications when selecting suture material to primarily close incisions when material such as Vicryl loses the majority of its strength at 1 month. Sutures are used to close gaping wounds, prevent hemorrhage and infection, support wound strength, and provide an aesthetically pleasing result. The major delineations between materials are monofilament versus multifilament and absorbable versus nonabsorbable. The biomaterial characteristics of different suture material and the time for which tensile strength is no longer needed in wound closure is important for choosing the best suture material. One of the basics is the desire to close the wound with suture that will maintain its strength until the wound itself begins to plateau on the tensile curve (4–5 weeks). This time varies—less for mucosal surfaces and more for areas of tension—hence the need for differing suture materials for these areas.
Cytokines in Wound Healing
Cytokines are important signaling molecules that are essential to the healing process. Our current understanding of wound healing has shown the important effects of cytokines; however, most of our knowledge is from in vitro studies. More than 30 cytokines are involved in wound healing produced by macrophages, platelets, fibroblasts, epidermal cells, and neutrophils. Our ability to predict and modify the expression of these cytokines has been much the focus. Future skin scar research is active, especially in the role of transforming growth factor (TGF)-β and the different subtypes that can either impair healing or accelerate healing. The key to the clinical applications of this research will be the cytokine delivery method and the timing of application.
Basic Tenets of Wound Healing
To optimize wound healing there are several basic tenets that one can follow. Winter, in studying epithelialization in pigs, noted 3 critical factors important for the healing of cutaneous wounds:
- 1.
Wound hydration
- 2.
Blood supply
- 3.
Infection minimization.
The ability of ointments and occlusive dressings to trap moisture in the wound has been shown to double the speed of epithelialization. This process prevents desiccation of the upper dermis and allows for more rapid epithelialization. A rolled wound edge is the clinical indicator that epithelialization has been halted. By freshening the wound edge, it helps stimulate and transform the quiescent epithelial cells into migrating keratinocytes to travel over the wound bed. The epithelial layer serves as a physical barrier to prevent infection and desiccation.
Intrinsic and Extrinsic Factors in Wound Healing
Factors affecting wound healing can be divided into intrinsic and extrinsic factors ( Box 1 ). Intrinsic factors are those related to the overall health of the patient and any predisposing factors. In general, any connective tissue and inflammatory disorders influence wound healing, such as in any patient with alterations in the key components of wound healing including leukocytes, protein production, and relative or actual immunodeficiencies (human immunodeficiency virus, diabetes, and so forth). Other obvious factors that decrease wound healing are chronic or acute diseased states including liver disease, uremia, malignancy, sepsis, and shock. Patients affected by these conditions may have limited ability to mount an immune response, provide less oxygenation to healing tissues, or have a diminished nutritional supply for the healing process.







