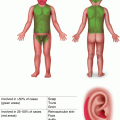
Fig. 11.1
Basal cell carcinoma on the nose of a teenage heart transplant recipient (image courtesy of Susan J. Bayliss, MD)
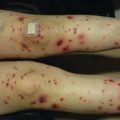
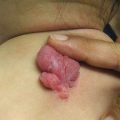
Voriconazole , a triazole antifungal , is effective at treating several infections, often those caused by Aspergillus spp. , but it is also active against Scedosporium, Fusarium, and Candida spp. [7, 8]. The Food and Drug Administration approved voriconazole in 2002, and since then many reports of photosensitivity and phototoxic reactions have surfaced. In 2010, a review of eight patients with 51 SCC after initiation of voriconazole therapy included two pediatric patients who each had a history of cord blood hematopoietic cell transplant and developed AK, SCC in situ, and SCC [9]. Voriconazole phototoxicity has several clinical appearances. The acute phase can include erythema (Fig. 11.2), blistering, erosions, scaling/desquamation, cheilitis, hyperpigmentation, and lentigines [9–12]. Long-term sequelae include lentigines, ephelides, atrophy, actinic keratoses (Fig. 11.3), and skin cancers, most often SCC [13]. Additionally, voriconazole phototoxicity can mimic a flare of GvHD [10]. SCC has been reported in pediatric cancer patients and HSCT recipients [9, 13–15], POTRs [13, 16], and immunosuppressed pediatric patients [13, 17]. The mechanism leading to voriconazole-associated SCC is unclear. Recent work proposes an increased risk for voriconazole-associated SCC in patients with ultrarapid metabolism of the drug, seen in the CYP2C19*17/*17 genotype [18].
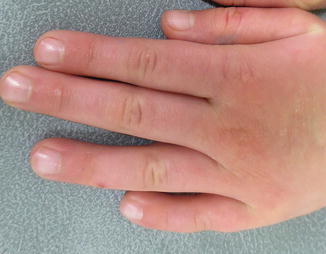
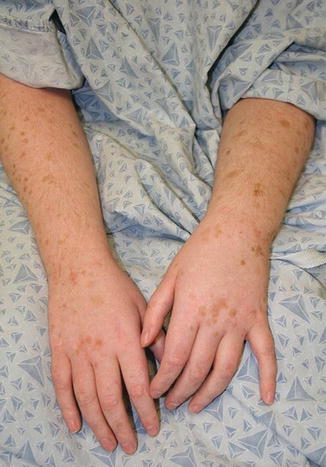

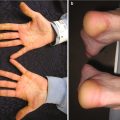
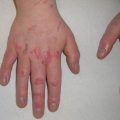
Fig. 11.2
Erythema and scale of the hand with voriconazole phototoxicity

Fig. 11.3
Solar lentigines and actinic keratoses in a child after long-term use of voriconazole (image courtesy of Jennifer T. Huang, MD)
Posttransplant Lymphoproliferative Disorder
Posttransplant lymphoproliferative disorder (PTLD) is the most common malignancy following renal transplant in children [5]. Its presentation in the skin, however, is rare. In a review including 16,130 patients with solid organ transplants from 1987 to 2008 who were <20 years old, the risk for both T- and B-cell lymphomas was elevated posttransplant [19]. The number of skin lymphomas in children captured in this study was very small, but cases of primary cutaneous anaplastic large-cell lymphoma (ALCL) and mycosis fungoides/Sezary syndrome were reported. With <3 cases of each, it is difficult to interpret significance, but given the rarity of primary cutaneous ALCL in children (see Chap. 3), the reports are notable. The B-cell diseases of Burkitt lymphoma/leukemia (24 cases; standardized incidence ratio [SIR] 123) and diffuse large B-cell lymphoma (138 cases; SIR 379) were much more common. This study did not comment on the frequency of cutaneous presentations of the diffuse large B-cell lymphomas, but skin involvement in this lymphoma has been reported in an infant (two reports of the same case) [20, 21]. Follicular mucinosis was reported in a 17-year-old patient with a history of acute myeloid leukemia, HSCT, and quiescent cutaneous GvHD [22]. At the time of the report there was no transformation to PTLD. The folliculotropic subtype of mycosis fungoides as PTLD has been seen [23]. Time from transplant to presentation varies, but many cases of PTLD (regardless of subtype) occur in the first year after transplant [19, 24]. Posttransplant B-cell disorders are often related to Epstein-Barr virus infection , which many attribute to the high incidence in children.
Kaposi’s Sarcoma
In children, Kaposi’s sarcoma (KS) typically presents in those who are immunocompromised, including POTRs and patients infected with the human immunodeficiency virus (HIV). It is associated with human herpesvirus-8 infection (HHV8; also known as Kaposi sarcoma herpesvirus ), but HHV8 infection alone does not cause disease. Impaired T-cell immunity in HHV8-infected individuals has been implicated in the disease pathogenesis [25]. Its incidence is markedly elevated in patients in or from sub-Saharan Africa infected with HIV [26]. Cutaneous involvement (such as patches, plaques, and nodules) is variable, but can occur in all types (epidemic, endemic, iatrogenic, and classic) of KS [25]. Classic KS in pediatric patients is uncommon [27]. POTRs may develop KS, but it is not common [5, 28]. See Chap. 9 for more details on HHV8.
Permanent Alopecia
Key Points
Permanent alopecia is common after cancer therapy.
Patients with higher cranial radiation doses are at increased risk to develop permanent alopecia.
Both traditional and targeted medical cancer therapies can predispose to the development of long-term alopecia.
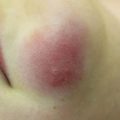
Permanent alopecia can be a distressing long-term side effect of cancer therapy, occurring secondary to radiation or medical therapies. Alopecia is considered permanent in this setting if it lasts for more than 6 months following the completion of therapy (Fig. 11.4). It can be diffuse or patchy. It is a commonly reported long-term side effect, though Casagranda et al. note that alopecia, along with other late effects, may be underreported by patients [29].
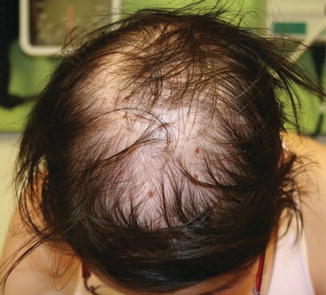

Fig. 11.4
Permanent alopecia present more than 20 years after radiation therapy as a child (image courtesy of Jennifer T. Huang, MD)
Radiation with traditional photon beam treatment , as well as proton beam, can cause permanent alopecia. In a study of adult patients, increasing mean follicle dose (dose calculated to contact the hair follicles) was associated with higher risk for permanent alopecia [30]. In this population (26 patients) treated with traditional photon beam cranial irradiation, the dose at which 50% of patients developed permanent alopecia was 43 Gy, and was not affected by chemotherapy agents the patients also received. In a Dutch cohort, childhood cancer survivors who received cranial radiation therapy more commonly experienced alopecia than those who did not [31]. Other work has shown alopecia incidence to be related both to radiation and chemotherapy. Specifically, in a group of 12 pediatric patients treated with proton beam radiation, patients who received standard-dose chemotherapy and 30 Gy of proton beam craniospinal radiation were at risk for permanent alopecia, while patients who received high-dose chemotherapy were at risk after receiving 21 Gy of proton beam therapy [32].
Traditional chemotherapy, especially high-dose regimens followed by HSCT , can cause permanent alopecia. In adults, busulfan-containing regimens have long been implicated [33, 34], though regimens without busulfan can also trigger alopecia [35, 36]. Busulfan has also been identified as a risk factor for permanent alopecia in pediatric patients [37–39]. ThioTEPA has been associated with permanent alopecia in some reports, both in children [40] and in adults [36] undergoing HSCT. In HSCT patients, GvHD is another significant risk factor for permanent alopecia [38].
Permanent alopecia, generally scarring, as a consequence of a pustular eruption with EGFR inhibitors has been reported in adults, particularly women [41]. Data about permanent alopecia in children taking EGFR inhibitors is lacking.
Alopecia is an unwanted and stress-inducing side effect for many patients and parents [38, 42]. Permanent alopecia has been associated with depressive symptoms in females [43]. Interestingly, it was associated with impairments in physical function, bodily pain, and general health, in addition to social function, vitality, role function, and emotional health on Short-Form 36 (SF-36) subscales [43]. Further work is needed to more completely describe the effects of permanent alopecia on cancer survivors’ long-term physical and emotional health.
Unfortunately, treatment options for patients with permanent alopecia are limited. Surgical procedures are complicated and invasive, but can provide some patients with good results [44]. Preventing alopecia would be a more ideal solution. Recent investigations into preventing acute alopecia in adults undergoing chemotherapy have utilized scalp-cooling devices, as well as pharmacologic agents [45, 46]. Long-term data, and data in children, has not been published yet.
Autoimmune Disorders
Key Points
Vitiligo and alopecia areata are the more commonly reported cutaneous autoimmune diseases following HSCT.
Many patients with autoimmune sequelae after HSCT also have GvHD.
Autoimmune sequelae of cancer therapies are not well described. Recently there have been several reports of cutaneous autoimmune disorders after HSCT. These can occur with or without a known donor history of the autoimmune disorder. This section focuses on vitiligo and alopecia areata after transplantation.
Vitiligo after HSCT occurs in both children and adults, presenting months to years after transplant. Both pediatric [47] and adult [48–50] patients have developed vitiligo after transplant from donors with a known history of vitiligo, but many reports of vitiligo after HSCT do not include this history. Children with history of cancer, primary immunodeficiency, and hemoglobinopathy have developed vitiligo, both after manifesting GvHD and without GvHD [51–55]. Interestingly, in a series of pediatric and adult patients with vitiligo and GvHD, three patients had disease after an autograft [54]; other series report patients with allografts. Some adult patients have had donor lymphocyte infusions prior to onset of vitiligo [56]. The convergence of total leukoderma and leukotrichia in patients with a history of HSCT is striking, and several cases of affected children have been reported [57–59].
Alopecia areata is less commonly reported as a consequence of cancer therapy. It has been seen as adoptive from the HSCT donor in adult cancer survivors [60, 61]. It has also been reported de novo in a handful of patients with GvHD, including in a 19-year-old male with history of HSCT for chronic myelogenous leukemia [62].
Patients with both vitiligo and alopecia areata in the setting of HSCT have also been reported [63]. In a series by Zuo et al. of patients with chronic GvHD and vitiligo and/or alopecia areata, 3/15 were under 18 years of age. Interestingly, though all had GvHD, not all had cutaneous GvHD [64]. Čeović et al. reported 10/50 patients with GvHD also developing vitiligo or alopecia areata, but did not specify which patients were children [65].
Several authors have debated the mechanism for autoimmune sequelae after HSCT, outside of adoptive transfer from donors, including donor recipient mismatch in female-to-male transplants, effect of conditioning regimens, genetic predisposition, and environment [64, 66]. More studies are needed to investigate these theories.
Scars
Key Points
Scarring is very common in childhood cancer survivors.
Scarring can affect mental health and quality of life in cancer survivors.
Treatment of scars in pediatric dermatology is evolving.
In the course of cancer treatment, patients have many exposures that can lead to the development of scars. Intravenous access can lead to scarring; peripheral and central lines, as well as ports, have associated scars. Additionally, complications of these access points, such as extravasation injury, can predispose to further scarring. Procedures such as biopsies (particularly skin and bone marrow) and resections are more obvious causes. Also, radiation therapy is a risk factor for scarring. In a review of 14,358 survivors of childhood cancer through the CCSS, scarring or disfigurement was reported by 25% of patients on the head or neck, 18% on the arms or legs, and 38% on the chest or abdomen [43]. On follow-up questionnaires, patients were queried on quality-of-life measures. Increased depressive symptoms were reported by patients with head or neck and arm or leg scarring or disfigurement. These patients also reported impairment in SF-36 subscales of general health and vitality, with mental health being affected in patients with head or neck scarring or disfigurement. Patients with arm or leg changes also reported impaired physical function, bodily pain, and social function. Thus, scarring is present long-term, and is associated with impairment of quality of life.
This data shows an opportunity for providers to be thoughtful in approaches to care and prevent or hide scars when possible. To this end, Braam et al. investigated prophylactic use of silicone sheeting for patients after removal of venous access devices (ports) [67]. The investigators showed nonsignificant improvement in patients’ scars after 2 months of use, but wider scars after 6 months of use. Thus, longer term use of the sheets cannot be recommended at this point, though short-term use could potentially be helpful. Scar treatment in pediatric patients has been evolving. In addition to the traditional options of surgical scar revision and, for thick scars, intralesional steroid injections, laser treatment with full ablative, fractional ablative, and pulsed-dye technology has been advancing. Laser-assisted delivery of medications to augment scar treatments is also progressing [68]. Thus, patients will have more options going forward for scar revision/treatment. Currently there are multiple validated instruments for patient-reported scar outcomes, developed through work with different patient populations such as dermatology, burn, and postsurgical patients, though each has drawbacks [69, 70].
Education and Anticipatory Guidance
Education about skin cancer risk and photoprotection is important for pediatric cancer survivors and other patients at risk for photosensitivity and skin cancers. There are several tools and websites that can help with this education (see Table 11.1). Of note, given the increased risk for skin cancer in users of indoor tanning beds [71–73], these devices should be strictly avoided in children [74, 75], especially in cancer survivors and other children more at risk for developing skin cancer. Childhood cancer survivors have incomplete adherence to photoprotection [76, 77] and skin surveillance [78] recommendations. Both children and their caregivers must be educated about skin cancer risk and photoprotection. Studies in both childhood cancer survivors and POTRs have shown improvement in short-term (1–6 months) photoprotection behaviors after education interventions [79, 80]. Importantly, education about these topics often needs to be repeated for patients and parents. In a study of POTRs and their parents, more than half of participants desired at least yearly reminders [81].
Table 11.1
Resources for pediatric photoprotection and skin cancer information and handouts for patient education
Organizations | Tool | Description | Source |
|---|---|---|---|
AAD: American Academy of Dermatology | General skin cancer education | Information about skin cancer and its prevention, as well as consequences of tanning bed use | |
CDC: Centers for Disease Control and Prevention | Skin cancer and sun education | General skin cancer and sun education, with a link to handouts for families and students | |
COG: Children’s Oncology Group | Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers | General guidelines for pediatric cancer survivors, with a section dedicated to skin | |
PeDRA: Pediatric Dermatology Research Alliance | Pediatric skin cancer handout | 2-page handout detailing risk, detection, prevention, and treatment of pediatric skin cancer | |
SPD: Society for Pediatric Dermatology | |||
AAP: American Academy of Pediatrics | |||
US EPA: United States Environmental Protection Agency | Pediatric sun safety handouts | Activities, fact sheets, handouts reviewing sun safety
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|