POLYMERS, & COPOLYMERS
Authors
Anthony J. O’Lenick, Jr.
President
Siltech LLC
2170 Luke Edwards Rd
Dacula, Ga 30019
Thomas O’Lenick PhD
Technical Director
SurfaTech Corporation
1625 Lakes Parkway
Suite N
Lawrenceville Ga 30043
Meyer R. Rosen, FRSC, CPC, CChE, FAIC
President
Interactive Consulting. Inc
P.O. Bo 66
East Norwich, New York, 11732
ABSTRACT
Silicone polymers have been known since 1880, however they did not become a major ingredient in the personal care industry until the 1990s. The designation “silicone polymers,” as will be seen in this chapter, covers a variety of silicon-based materials that are characterized by molecules or polymers that contain the Si-O-Si bonds. For convenience, and ease of introducing these materials to you the reader, we have initially simplified our speaking about these materials to “silicone polymers.” However, one of the goals of this chapter is to distinguish that “silicone polymers” actually represent a whole range of materials ranging from simple silicon-based molecules (e.g., silanes) to “straight” polydimethyl siloxane polymers (of varying molecular weight to linear and cyclic variations of these; to “organosilicone” copolymers of different chemistries (like “T” configurations or “ABA” type polymers; to cross linked variations of silicon-based materials which vary in the degree of cross-linking to generate yet another class of gelled “silicone polymers” of varying degrees of rheological behavior and applications in the personal care and cosmetic industries. Further, as we delve deeper into this enormous and widely useful class of materials in the cosmetic and personal care industry, one of our intentions is to make sure that the “old” use of the term “silicone copolyol” (from its initial INCI definition), disappears forever since there is no one such material as “silicone copolyol!”
We also address the apparent trend away from “silicones” in light of their reputation as useful, but not “natural”—and how the technology is evolving to deal with this issue without losing the unique properties the materials are famous for. Further, the chapter addresses the potential toxicity question of very low molecular weight cyclic silicones, which provide a unique, noncooling effect on evaporation and alternatives to these cyclic silicones known as cylcomethicone (D4 and D5).
4.2.3.14 Silicone Nomenclature
a. Summary of Silicone Polymer Structure Types
a. Cyclomethicone Replacements
b. Summary of Successful Replacements for Cyclomethicones
d. Ultra-High-Viscosity Fluids (Gums)
e. Summary of Silicone Polymer Behavior
4.2.3.17 Resins and Elastomers
4.2.3.18 Dimethicone Copolyol (PEG/PPG Dimethicone)
a. Wetting Properties as a Function of Molecular Weight
b. Eye Irritation as a Function of Molecular Weight
c. Formulation Ingredient Interactions
b. Silicone-to-alkyl ratio effects
4.2.3.20 Multi-Domain Alkyl Dimethicone
a. Syneresis Improvement with Multi-Domain Silicones
4.2.3.21 Alkyl Dimethicone Copolyol
4.2.3.22 Greening with Silicone
Silicones have become ubiquitous in our industry and, in fact, there are few product categories that do not have “silicone” present. This fact has been made abundantly clear by the rapid growth in the number of patents related to silicone ingredients and their compositions. We also note their use in formulations for numerous cosmetic and personal care categories and the many synergistic effects obtained with other components.
There are a few key, unusual functional attributes that make silicone a “must have” in a personal care formulation. These include: better surface tension reduction than that obtained with organic surfactants and, at much lower concentrations; as well as the capability of the forming associative films upon dry-down. Further, in view of the presence of the silicone moiety, these materials have expanded emulsification properties beyond those of organic surfactants. They also have foaming ability in both aqueous and nonaqueous environments the capability to disperse pigments, and an ability to generate a desirable, unusual sensory/aesthetic silky feel.
As we have stated above, silicones provide an outstanding feel and unparalleled sensory performance that a formulation would be lacking without the presence of silicone. This chapter addresses the selection process, which is drawn from a host of molecular choices that vary in their structure and stereochemistry.
Silicones can drastically change the performance of a cosmetic formulation by the modification of two salient properties: (1) insolubility in both hydrocarbon oils and water, as well as (2) surface and interfacial properties when hydrophilic and hydrophobic moieties are present. While these two properties seem simple enough, a good understanding of the two, and the value they bring to formulations, is key to the proper selection of organosilicone polymers in order to obtain specific desirable properties. In short, silicone polymers should be used in a formulation only when they provide benefits not provided by other classes of polymers.
Today, the global consumer is faced with many often-contradictory desires when selecting a cosmetic or personal care product. The cosmetic industry is driven by the consumer in the sense that if a cosmetic formulation does not feel good on topical application, they will not buy it no matter how well it performs. As we enter the “Green Chemistry” age, consumer demands have evolved and changed towards use of natural products. The desire for formulations that are “green” has become a major focus when formulating a cosmetic formulation. While the definition of green chemistry has been debated and will be addressed later in this chapter, one thing is clear: Consumers are looking for products that are “green,” and contain a greater percentage of sustainable raw materials. This push towards green products has some limitations; none bigger than the fact that while the consumer wants a green product, the demand for cost-effective performance still remains. This dichotomy, and schism, makes the proper selection of silicone-based raw materials even more critical. It is commonly accepted that silicone is not green. This leads to the concept that silicone compounds should be incorporated into cosmetic formulations in minimal concentrations. Essentially, formulations utilize silicone to drive performance upwards and not as a main ingredient. This means that judicial selection of the most efficient silicone will be used in formulations, and silicone- based products will be used only when other less costly options are unavailable. Determining the efficiency of silicones in formulations is an absolute requirement, and this chapter provides insights on methodologies to fine-tune formulations and achieve the optimal balance between perception, cost, and performance.
It is important to understand that the term “silicone polymer” refers not to one material but to many classes of products. The simplest class is a polymer that contains only dimethylpolysiloxane, and this term generally refers to the term “silicone fluids.” Other classes of silicone polymers contain organic groups besides, or in place of the methyl groups and are referred to as organo-functional silicone polymers. One of the unique properties of silicone fluids is the fact that they are insoluble in both water and oil (Figure 2.0.1). Since oil and water are two of the most common ingredients in cosmetic formulations, how they interact with the silicone polymers is a significant key to formulation performance.

Figure 2.0.1: Solubility
This difference in solubility from both water and hydrocarbon oil has long been an invitation to the chemistry community to rethink the terms “hydrophobic” and “hydrophilic.” In the cosmetic and personal care world, in which there are primarily only two immiscible phases, oil and water, the terms hydrophilic and hydrophobic are sufficient. In fact the whole concept of hydrophilic/lipophilic balance (HLB) by Griffin (discussed at http://en.wikipedia.org/wiki/Hydrophilic-lipophilic_balance) has been eminently useful to describe the behavior of “organic” surfactants.
When the silicone moiety is introduced, new terms become necessary. This is due to the fact that a hydrophobic material can be either oil-loving (oleophilic) or silicone-loving (siliphilic).i The term “group opposites” has been applied to this concept.ii Table 2.0.1 shows the classifications.
Solubility Characteristic Terms | |
Hydrophilic (water loving) | Hydrophobic (water hating) |
Oleophilic (oil loving) | Oleophobic (oil hating) |
Siliphilic (silicone loving) | Siliphobic (silicone hating) |
There are several new terms introduced in Table 2.0.1. With these distinctions, materials can now be broken down into more specific categories. A hydrophobic (water-hating) product can be either oleophilic or siliphilic. Oleophobic (oil -hating) materials may be either hydrophilic or siliphilic. Siliphobic (silicone-hating) materials may be either oleophilic or hydrophilic. If a hydrophobic or siliphobic fiber is treated with “silicone” or with a hydrocarbon, the fiber will become oleophobic at the surface. Thus, for application in waterproofing fibers, selection of the proper solubility characteristics is critical.
The other unique and salient characteristic of silicone compounds, either silicone fluids or silicone polymers containing other functional groups (i.e., organo-functional) is the fact that silicone-containing polymers have much lower liquid/air surface tension as compared to hydrocarbon oils and water. Surface tension has been defined as: “the energy required to break through the surface of a substance.”iii In a bulk solution, the molecules at the air/solution interface behave differently than a molecule in the bulk solution. This leads to superior surface-active properties. An interesting example of this behavior is that of water. Water, at the air/water interface has a high surface tension (72 dynes/cm), leading to a high boiling point (100°C) and the ability to suspend a paper clip gently placed upon its surface! This high boiling point is surprising because of the low molecular weight of water (18 g/mol). The major reason why the surface tension of water is so high is because of hydrogen bonding. When compared to hydrocarbon oils and water, their surface tension is significantly lower than that for silicone. Table 3.0.1 shows typical surface tension values.
Surface Tension | |
Material | Surface Tension (dynes/cm) |
Mercury | 472.0 |
Water | 72.6 |
Squalane | 46.2 |
Soap Solution (1%) | 38.8 |
Mineral Oil | 33.1 |
Dimethicone (i.e., polydimethyl siloxane)(20cP) | 26.6 |
Acetone | 23.7 |
Hexamethyldisiloxane | 22.7 |
Ethanol | 22.2 |
Cyclomethicone (cyclic silicone with 4 repeating units of O-Si-O(D4) | 20.6 |
Diethyl ether | 17.0 |
The low surface tension demonstrated by polydimethlysiloxane relates directly to its structure, how the molecules orient at the liquid/air surface, and the intermolecular forces. Silicone fluid is a silicone polymer consisting of repeating units of Si-O [–(O-Si(CH3)2)n-]; its structure is shown below (Figure 3.0.1).
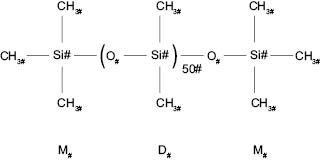
Figure 3.0.1: Structure of Silicone Fluid (Polydimethylsiloxane)
Silicone fluid (polydimethylsiloxane) does not have strong intermolecular forces, and the only intermolecular forces it possesses are dispersion forces that refer to their superior ability to spread out on surfaces. Furthermore, the silicone polymer has very flexible Si-O bonds in the polymer backbone, and is highly branched due to the methyl groups. This branching affects the way the polymer chains interact with one another. The presence of methyl groups on the polymer prevents chains from packing tightly, thus decreasing surface tension. Figure 3.0.2ii shows a computer-generated structure for a silicone fluid. The polymer is known to coil up on itself, much like DNA, and this may be visible, somewhat, in Figure 3.0.2 below.
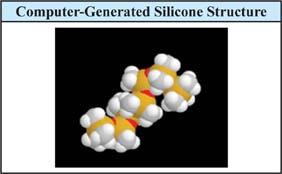
Figure 3.0.2:ii Silicone Surfactants (Organo-Functional Silicones)
Surfactants are a class of compounds commonly used in cosmetic formulations. An extensive discussion of surfactants is presented elsewhere in this text. Surfactants have been defined as: substances that change the properties of a surface.3 When a surfactant is added to a solution in which it has some solubility, it migrates to the air/liquid interface. This migration causes a disorder in the surface wherein the hydrophobic portion is ejected from the liquid (water), thus decreasing surface tension since the interface now appears hydrophobic. Below is a graphic of how surfactants lower the surface tension of a material.5 The graphic covers a range of surfactant concentration from below the critical micelle concentration (i.e., not enough to entirely cover the air/liquid surface) to higher levels where the surface is filled and excess surfactant is pushed under the surface, migrate to each other and form “micelles,” which are clumps of surfactants with their outside consisting of the surfactants’ hydrophilic portion and the inside of the micelle consisting of their hydrophobic portions.
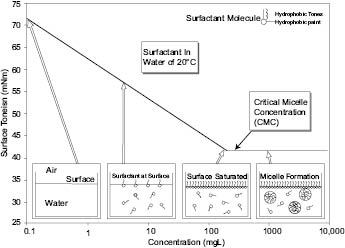
Altering the surface tension of materials provides many of the desirable properties that silicone copolymers bring to cosmetic products as outlined in Table 3.0.2.
Desirable Properties of Silicone Polymers |
1. Skinfeel |
2. Spread |
3. Film Formation |
4. Dry feel |
Further discussion of critical micelle concentration (CMC) and how silicone affects the surface tension of a bulk solution will appear later in this chapter.
4.2.3.14 SILICONE NOMENCLATURE
Silicone fluids are polymers made up of building blocks or monomers. The word “polymer” can be broken down into “poly” meaning many and “-mer” meaning unit. Typically silicone polymers are is made up of repeating silicone oxygen bond units. These “repeat” units are called monomers and they have to be at least di-functional. When these monomer units react with themselves they can either form a linear polymer chain (silicone fluid) where the more of them that react, the higher the molecular weight (into the millions for silicone rubber), or rings that are coined (cyclics). Cyclics are essentially polymer chains that form when one end group reacts with the other, forming a ring. There are several common building blocks, so named by how many silicon-oxygen bonds they contain. Figure 6.0.1 below contains a list of the most common building blocks used in silicone polymers.6 The figure also shows a shorthand designation for each structure that is quite useful in describing these complex materials: M, D, T, and Q.
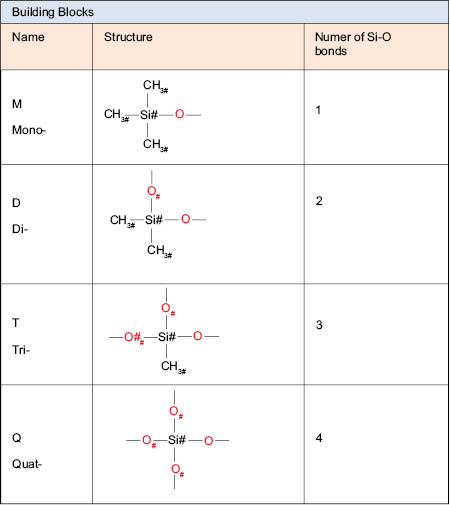
As seen in the table, each building block is named by how many silicon-oxygen bonds it has; “M” units or mono-substituted groups are “chain terminators.” They only have one reactive site, so they have to be on the end of the polymer chain, or as a pendant group of the silicone polymer chain. Pendant groups are groups that “hang” off of the main polymer backbone or polymer chain. M units cannot be in the silicone polymer backbone because they only possess one reactive group. Unlike the M unit, the D unit is di-substituted and will be found in the polymer backbone. The T and Q units are used as cross-linkers, linking two or more polymer chains together. Typically, chemists refer to silicone polymers as silicone fluids and identify their structures by their letters and viscosities. An example of this is MD50M. The structure of this polymer is shown in Figure 6.0.2.
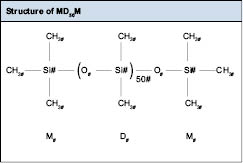
MD50M is a silicone polymer made up of 50 D units, end-capped with M units. It is synthesized by the reaction of MM with cyclic D4 or D5. A detailed discussion of cyclo-silicones and fluids will appear later in this chapter.
It must be clearly understood that the chlorosilane materials made in the Rochow process result in the building blocks that are found in the polymers and for which the nomenclature is derived.
All of the units mentioned so far are useful for synthesizing silicone fluids or nonfunctionalized (e.g., with organic groups, for example) silicone fluids. To functionalize silicone polymers, new building blocks with reactive sites are needed. Figure 6.0.3 outlines some of the common reactive building block sites. These are stereochemical positions created by the basic starting structure where other reactive materials can be attached. One example of this would be a short hydrocarbon chair, or a short chain of polyethylene glycol or, getting more complex, low-molecular-weight copolymers (either random or block) of ethylene oxide and propylene oxide.
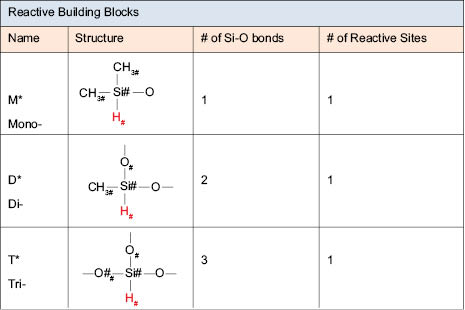
It is important to note that there is no Q* reaction site shown. The Q building blocks have four silicone-oxygen bonds and therefore they cannot have a reactive site bonded to them. However, even these can be reacted with M end blockers to make MQ resins, which also are another type of silicone gels that have found usefulness in cosmetics.
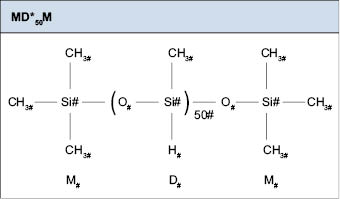
Silicone polymers can contain from one to several reactive sites. They are typically constructed by reacting either M*M* with a cyclic, a reactive cyclic with MM, or M*M* with a reactive cyclic. Reactive cyclics are typically denoted as D5*. They follow the same nomenclature as above. If we take the polymer from above (MD50) and add reactive sites, its name gets changed to MD*50M. The structure is shown in Figure 6.0.4.
These reactive sites can be placed at three parts of the polymer backbone. They are named after what the polymer resembles following the reaction. Figure 6.0.5 shows illustration of the three common structural types.
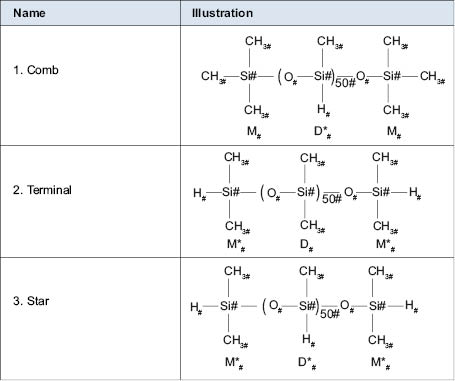
Theses three categories are so named by the theoretical shapes of the polymer backbones. The first category is called comb. This is because when the chemist draws out the structure it appears like a hair comb. All of the reactive sites are on one side (pendant) and along the backbone of the polymer or comb. It should be taken into consideration that the polymer backbone has free rotation and the reactive groups can be in many different arrangements when the polymer chain is in motion. The second category is so named terminal. This category is self-explanatory by the fact that the only reactive groups on the polymer are at the chain ends. The final category is the star category. This category is so named because it could be envisioned that this polymer chain would have reactive groups on all sides of it and at the ends. Such molecules grow outward from a center in a multiplicity of “arms.”
It is important to note that the functional group shown above is the Si-H bond. This is not the only reactive site used in the synthesis of functional silicones. There is a long list of functional groups that can be incorporated to fully utilize silicone chemistry.
a. Summary of Silicone Polymer Structure Types
The silicone backbone that is prepared by the technology developed by Rochow makes up what is called the construction of the silicone polymer. It is the number and location of the so-called Ds, Ms, Ts, and so on that determines one major part of the function of the polymer. The other parameter that helps define the structure-function behavior of these materials is called functionalization and is determined by what the M*s D* and the like are reacted with to make organo-functional silicones. There are indeed a great number of products that can be made using this technology.
Volatile silicones comprise a class of compounds that have a low heat of vaporization; that is, they evaporate from skin easily, providing a cooling effect. Evaporation of a material from the skin causes a cooling effect. Cyclomethicones conform to the structure shown in Table 7.0.1.
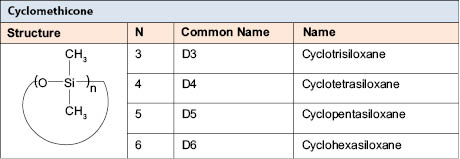
The INCI name “cyclomethicone” refers to a family of cyclic dimethyl siloxanes that includes cyclotetrasiloxane (D4), cyclopentasiloxane (D5), and cyclohexasiloxane (D6), a family that has come under increased environmental/regulatory scrutiny in recent years. Cyclomethicone compounds are commonly used in cosmetic products to provide a solvent that feels dry on the skin. Key areas where this family of materials is used include antiperspirants, color cosmetics, and as a base solvent to blend with fragrance oils and perfume oils. Cyclomethicone is a clear, odorless silicone that leaves a silky-smooth feel when applied to the skin. Cyclomethicone compounds possess a cyclic structure rather than the chain structures of linear dimethyl silicones (dimethicone). D4 evaporates from the skin and is volatile, resulting in a lower heat of vaporization, but it also has a very low surface tension. In other words, the volatility of D4 is only one of the reasons it has a dry skin feel, but its low surface tension resulting in spreadability on the skin cannot be ignored. However, the physical chemistry that results in the dry feel of cyclomethicone evaporating is a complex one. Volatility is but one aspect of the complex phenomenon that contributes to a dry feel in a solvent used in cosmetics. In view of the safety concerns associated with the low-molecular-weight cyclomethicones, replacements have been sought. These must also provide similar viscosity and surface tension reduction (which affects spreadability), and must be nonflammable and cost effective (a formidable combination!). Clearly, a D5 replacement that is flammable, defatting, and expensive is unacceptable. Table 4.2 shows the Heat of Vaporization of some compounds. Table 7.0.2 shows that it takes significantly less energy to evaporate D4 or D5 than either water or ethanol.
Heat of Vaporization | |
Materials | Heat of Vaporization (cal/g) |
Water | 539 |
Ethanol | 210 |
D4 | 31 |
D5 | 31 |
The cyclic silicones in Table 7.0.2 have a dry feel when applied to the skin, for two main reasons. First, they have the ability lower surface tension of the formulation, containing oil and thereby leading to a drier-feeling product, since they are surface active in oils. Second, since the cyclics are so volatile, they evaporate rapidly when they are exposed to the temperature of the skin. There are a number of cosmetic applications in which a dry skinfeel is important. These include antiperspirants, skin serums, and sun-care products. Table 7.0.3 shows some other characteristics of cyclic silicones.
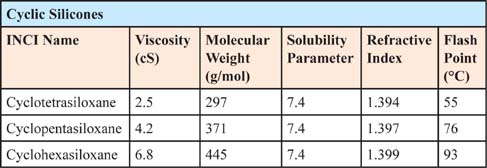
Recently, the use of D4 in personal care applications has been abandoned because of safety concerns. The most common replacement has been D5, and to a lesser extent, D6. While drawn from the same family, these materials have different physical properties and do not result in the same dry feeling as D4.
Cyclomethicone is used in a variety of products. From a commercial view, most importantly they have been used in antiperspirant formulations because they:
- (1) Impart a soft-silky feel to the skin
- (2) Provide excellent spreading
- (3) Leave no oily residue or buildup
- (4) Detackify formulations
- (5) Are nongreasy
- (6) Are compatible with a wide range of cosmetic ingredients
- (7) Lower surface tension and
- (8) Provide transient emolliency on the skin.
Cyclomethicones are used in many other applications including:
Table 7.0.4
Cyclomethicones in Cosmetic Application |
1. AP / DO |
2. Hair Sprays |
3. Cleansing Creams |
4. Skin Creams, Lotions |
5. Stick Products |
6. Bath Oils |
7. Suntan |
8. Shaving Products |
9. Makeup |
10. Nail Polish |
a. Cyclomethicone Replacements
As we have said, over the years, the cosmetic industry has been actively seeking cyclomethicone replacements. D4 is no longer used in our industry and despite some indications that the underlying conclusions that it is problematic in cosmetic products is faulty, the consumer simply will not accept these materials in formulation. The sharp consumer sensitivity to cosmetic formulation additives will also have an effect on D5. While it is guilt by association, it is my feeling that long-term D5 will also disappear. Products that contain large quantities of D5 will be first to feel the wrath of consumers. In fact, in the future the use of silicone in formulation will be limited to those polymers that (a) provide a function that is not attainable from other types of materials and (b) are effective at very low concentrations. Put metaphorically, if a cosmetic product is a gourmet meal, the silicone additives will be the spice to the meal and not the meat and potatoes.
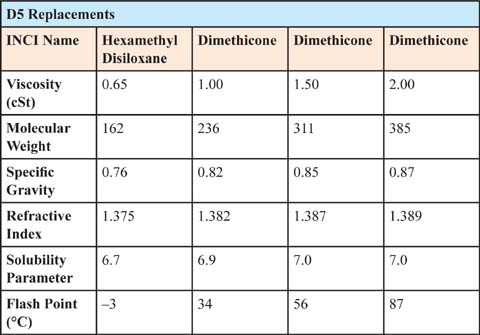
One approach to cyclomethicone replacement is to use low-molecular linear dimethicone. The most common linear polydimethylsiloxane (dimethicone) used to replace the cyclics range from 0.65 cSt to 3 cSt fluid. Despite the added cost, of these materials over that of cyclomethicone, they have enjoyed some success because of their similar properties. (See Table 7.1.1.)
Another approach to replacing cyclomethicone is to use a silicone that is a methicone and not a dimethicone. Dimethicone compounds contain a dimethylsiloxane group; methicone compounds contain only mono methyl silioxanes wherein the other methyl group is replaced with a non-methyl organo-functionality. These polymers are not made from D4. When a properly selected methicone is added to triglycerides or other esters, such a material: (1) will lower the surface tension at low concentration, making esters and triglycerides feel more like silicone; and (2) can be used in an ester or triglyceride at less than 5 percent, providing a cost-effective D5 like feeling.
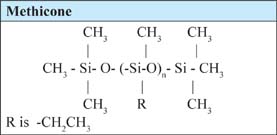
The desirable dry effect achieved on the skin by the efficient reduction of surface tension of a natural oil, or low-viscosity ester, by means of using low concentrations of ethyl methicone offers a valuable formulation tool for the formulator. Replacing D5 with combinations of natural oils and a small amount of alkyl silicone results in a replacement that is greener than D5 (due to the fact that natural oils are present). Table 7.1.2 shows an example of how D5 can be replaced by another silicone in an antiperspirant formulation.6
Antiperspirant Formulations8 | |||
Material | Formulation | ||
A | B | C | |
Part A | |||
Aluminum/Zirconium | 24.000 | 24.000 | 24.000 |
PEG/PPG 18/6 Dimethicone | 2.500 | 2.500 | 2.500 |
Part B | |||
Cyclopentasiloxane | 30.000 | 0.000 | 0.000 |
Ethyl Methicone | 0.000 | 30.000 | 1.500 |
Glycine SoJa (Soybean Oil) | 0.000 | 0.000 | 28.500 |
Part C | |||
Isohexadecane | 9.000 | 9.000 | 9.000 |
PPG-14 Butyl Ether | 9.000 | 9.000 | 9.000 |
Hydrogenated Castor Oil | 2.500 | 2.500 | 2.500 |
PEG-8-Distearate | 1.000 | 1.000 | 1.000 |
Stearyl Alcohol | 18.000 | 18.000 | 18.000 |
Part D | |||
Talc | 3.000 | 3.000 |
|









