.7
CHRONOBIOLOGY OF THE SKIN
SKIN CIRCADIAN RHYTHM AND CLOCK GENES:
A NEW APPROACH TO SLOWING DOWN
THE AGING PROCESS
Authors
Nadine Pernodet, Ph.D. Vice President of Skin Biology Research
Edward Pelle, Ph.D. Director, Skin Biology Research
Estée Lauder Research Laboratories
Melville, NY, US
ABSTRACT
It is now well established that our body follows a circadian rhythm, which means that it responds to day-night cycles. This multi-oscillatory network has made human physiology adapt to environmental changes and, importantly, helps us to adjust our internal clocks and achieve proper body function.
The master clock that regulates this 24-hour cycle throughout our body is found in the suprachiasmatic nucleus (SCN), in the part of the brain known as the hypothalamus. This area of the brain responds to light received through the retina of the eye. Recently, mammalian peripheral molecular clocks, also known as clock genes, have been shown to be present in nearly all the cells in our body. The clock genes synchronization is controlled by the suprachiasmatic nuclei (SCN) and is responsible for directing cells to do the right thing at the right time! This mechanism has helped the human species to survive and plays a critical role in genome stability maintenance.
More than 10 percent of our genes are controlled by this circadian cycle and synchronize many of our internal molecular processes. The group of genes under circadian control includes those involved in DNA damage-repair responses, cellular proliferation, cell cycle, cell-cycle arrest, apoptosis, and metabolism. Uncoupling of circadian gene expression from the cell cycle has been shown to induce genome instability, premature aging, and cancer, as well as causing an increased tendency towards diabetes and obesity.
The skin, as the largest organ of our body, also follows a circadian rhythm. Each skin cell contains clock genes that control and synchronize cellular activities for generation of optimal benefits to the skin. This chronobiological phenomenon shows that during the day most of the cellular energy is used to protect the skin while during the night most of the energy is used to restore, repair, recharge, and regenerate skin cells. Unfortunately, because of different factors (i.e., stress, aging) this well-orchestrated mechanism inevitably goes out of sync. In this chapter we review the benefits of understanding the skin circadian rhythm and the importance of maintaining it in optimal synchronism as long as possible in order to slow down the aging process. As such, this understanding provides opportunities for leads to new breakthroughs in anti-aging technology for the personal care industry
5.7.1 Introduction to Circadian Rhythm and Clock Genes
5.7.2 Desynchronization: Causes and Impact
5.7.1 INTRODUCTION TO CIRCADIAN
RHYTHM AND CLOCK GENES
It is now well established that our body follows a circadian rhythm, responding to daylight, which helps us to adjust our internal clocks. Anyone who has traveled long distances by airplane has observed how their body is still working well at the time of their departure but not in the arrival city. This phenomenon is a result of the fact that our internal clocks usually need a few days in order to readjust all of our biological functions so that our cells can function correctly at the right time!
Our circadian clock promotes metabolic efficiency by timing activities with day-night rhythms following the Earth’s rotation time over a 24-hour period. This clock gives optimal efficiency to each cellular function, which has helped living species to survive for eons. As a consequence of the Earth’s rotation every 24 hours, organisms are subjected to fluctuations in light and temperatures. There is an evolutionary relationship between our internal physiology and the geophysical earth cycle to help our body adapt to the daily cycle of day and night. The fact that we have biological clocks provides us with an adaptive advantage by ensuring that our body functions are optimally adapted to their environment. This pattern results in optimal physiological efficiency. Circadian rhythms and cycles control our sleep pattern, body temperature, heart activity, hormone secretion, blood pressure, oxygen consumption, metabolism, and many other activities. To paraphrase Edmunds (1): “The ‘right’ substance (molecule) should not only be at the ‘right’ place in the ‘right’ amount, but all of this should also happen at the ‘right’ time.” This well-orchestrated organization synchronized by clock genes under SCN control makes sure that all of these functions happen at the right times and repeat every day!
Nowadays considerable research is being directed toward developing an increased understanding of the circadian rhythm and our internal clocks. This intriguing phenomenon has clearly shown, when desynchronized or out-of-sync, to be responsible for certain diseases such as cancer and obesity. Rhythm generation is now understood to be an intrinsic property of single cells that are driven by an intracellular molecular oscillator based on transcriptional negative feedback loops responding to synchronization coming from the suprachiasmatic nucleus (SCN) in the hypothalamus located in the brain. The SCN is the master clock (Figure 1), the SCN that regulates this 24-hour cycle throughout our body and is composed of neurons that can sense external cues such as light and darkness transmitted through the retina. The human circadian rhythm is actually a little longer than exactly 24 hours and will be maintained even when the organism is removed from light. However, as soon as light is received by the retina again, the clock will reset itself to match the earth’s rhythm of 24 hours. This is because mammalian molecular clocks are not only located in the SCN but also in nearly all cells in our body.
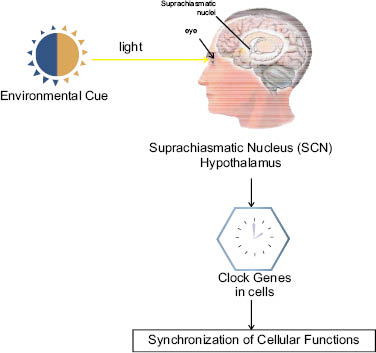
Figure 1: Circadian rhythm in the body is controlled by the suprachiasmatic nucleus located in the hypothalamus. Environmental cues such as light are transferred to the SCN, which then synchronizes all cellular functions in the different organs. The neuronal and hormonal activities it generates regulate many different body functions in a 24-hour cycle, using around 20,000 neurons.
Most cells and tissues express autonomous clocks. In the past few decades, scientists have discovered the genes responsible for running the internal clocks. Genes hold the information to build and maintain an organism’s cells and pass genetic traits to offspring. The human species has more than 20,000 genes encoded by DNA. A part of these genes is the Clock gene family. These include but are not limited to period (per), clock (clk), cycle (cyc), timeless (tim), and others (“Advances in Understanding the Peripheral Circadian Clocks,” Richards J, Gumz ML. FASEB J 2012; 26(9): 3602–13). The authors recognize that some readers may not be familiar in detail with genes and their behavior and we direct you to other parts of this book where a more detailed description of these important biological entities and their behavior are described.
A central player in the circadian cycle is the PER gene, which produces the PER protein. PER levels are at their highest during the early evening, and at their lowest early in the day. PER was the first clock gene identified, in 1971 in fruit flies. In 1997, the first mammalian clock gene Clock (Circadian locomotor output cycles kaput) was discovered in mice. Scientists found its location and decoded its DNA sequence. In 2001, Fu et al. (2) discovered the first human clock gene. At the molecular level, circadian rhythms are encoded by an autoregulatory loop composed of a set of clock gene proteins that act as transcriptional factors. The best-characterized of these are CLOCK and BMAL1, which induce expression of its repressors (PER1-3/CRY1-2) that feedback to inhibit the forward mechanism. This is illustrated in Figure 2. The transcription factor dimer CLOCK/BMAL1 drives expression of the target genes periods (PER1-3) and cryptochromes (Cry1-2) by binding to E-box elements in their promoters. The negative feedback comes from the PER/CRY protein complex that moves back to the nucleus, where it blocks CLOCK/BMAL1-mediated transactivation, thereby inhibiting its own transcription. In a second feedback loop, the nuclear receptor REV-ERBα rhythmically represses BMAL1 transcription.
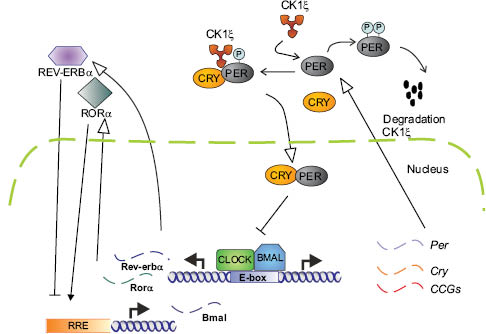
Figure 2: The mammalian circadian clock. CLOCK and BMAL1 form a heterodimer that binds to E-boxes in regulatory regions to activate transcription of target genes such as PER and CRY. Used with permission, Journal of Investigative Dermatology, Geyfman and Andersen (3).
Other genes activated by CLOCK/BMAL1 are referred to as CLOCK-controlled genes (CCGs). These include the D-box binding protein (DBP), REV-ERBα, and RORα, which are responsible for the oscillation of BMAL1 mRNA by repressing and activating BMAL1 transcription, respectively, by binding to ROR response elements (RRE). The time lapse between transcription of PERs and CRYs and nuclear entry of their gene products, as well as regulated degradation of clock components, is responsible for the oscillation of circadian clocks. Casein kinase (CK) phosphorylates PERs, thus regulating both nuclear import and degradation (3).
To date, 10 to 20 percent of genes in the body have been shown to follow a circadian oscillation, thereby allowing cellular functions to occur at the right time for optimal results and efficacy. The group of genes under circadian control includes those involved in: DNA damage repair response, cellular proliferation, cell cycle, cell-cycle arrest, apoptosis, and metabolism. In view of the synchronization of cellular activities in different tissues, it is now well accepted that different treatments can have different levels of efficacy based on what time of day they are applied or administered. For example, clinical studies have shown that there is an optimal time of day for the delivery of chemotherapy (4–6).
5.7.2 DESYNCHRONIZATION: CAUSES AND IMPACT
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








