MBA in Management and Finance
Senior Vice President Sales and Marketing
Barnet Products
140 Sylvan Avenue
Englewood Cliffs, 07632 NJ
ABSTRACT
It is well known that whitening products represent a very large category for skin care in Asia , especially in Japan where beauty products feature the function of Bihaku (beautiful white). Eastern women tend to have yellowish skin, so whitening in Asia is equivalent to anti-wrinkles for Caucasians. A light complexion was considered as a sign of elegance among nobles, educated people, and members of the higher classes in China since ancient times. As far back as the ancient Qin Dynasty (221–205 bce), there have been records on Chinese medicines that have beauty functions. For example, there were records about using Bai Zhi (Angelica dahurica) in facial cream during this period. In the later Tang Dynasty skin care grew in popularity.
Like the women in ancient China, women in ancient Japan were also concerned about maintaining a white skin. No wonder that the faces of Japanese women are white in the drawings and paintings. More recently, in the last half-century, there appeared the first whitening powders in Japan and 20 years ago such products became a real boom. Along with this evolution came some regulation such as Quasi-Drug in Japan but also more sophisticated mechanism-based products beyond the classic anti-tyrosinase activity. This chapter looks at the evolution of the approach to whitening in the last 50 years and then explains the mechanism of action of the different active families and specific ingredients within those families. It also covers lightening of dark spots and moves from the basic molecules through more and more complex molecular ingredients and finally to modalities and possibilities based upon molecular biological approaches.
4.2.4.1 Historic Evolution of Whitening Products in Japan
4.2.4.2 What Is a Whitening Quasi-Drug?
4.2.4.3 What Are the Quasi-Drug Additives?
c. – In the nerves—a new approach to the reduction of dark spots
4.2.4.1 HISTORIC EVOLUTION OF WHITENING
PRODUCTS IN JAPAN
In this chapter we present a unique view of the evolution of the category of skin whitening from the point of view of company and product introductions during each decade beginning with the 1960s.
In the 1960s, the first whitening powders appeared in Japan, for example, Soie de Reine Beauty C powder by Kanebo in 1966. The product contained an oil-soluble form of vitamin C and the goal was to provide a safe alternative to the bleaching action of hydrogen peroxide.
In the 1970s, the first whitening product series appeared with the introduction of Real Beauty (Kanebo) and Natural Shine (Albion) in 1971, and Freshure (Shiseido) in 1976.
In the 1980s, new lightening ingredients were developed and used in Lumiera (Pola) in 1980; Faircrea (Kanebo) in 1984 and in 1985 in UV White (Shiseido) and Sekkisei (Kose). This is also the time where the product form changed from a powder to an essence introduced by Estée Lauder (Swiss Whitening) in 1986, Kose (Sure White) in 1988—the year when Sansho Seiyaku obtained regulatory approval by the Japanese Ministry of Health, Labour, and Welfare (MHLW) to use kojic acid as quasi-drug (a short list of regulated actives used by skin care companies to use the claim “whitening”)—and Kanebo in 1989 (Kanebo Blanchir). An essence corresponds to a serum in occidental countries: a water-based thin emulsion.
In the 1990s the skin-whitening category began to boom and extend its reach to the mass market as younger customers were targeted. Shiseido launched Whitess Essence (with arbutin), Kanebo launched Freshel White C (with “penetrating” vitamin C), and Kao launched Shokubutsu (with vitamin C as well). Closer to the year 2000 the lines were again altered to new forms of products such as wipe-off lotion-type and soap-type products for cleansing, which performed by removing old keratinocytes.
After creating new type of products in the beginning of the 21st century, the trend has been to create more exclusive quasi-drug (QD) actives for lightening skin. This approach was accelerated in 2001 due to the BSE (Bovine Spongiform Encephalopathy) issue. The largest companies: Kose, Lever, Pola formulated out the bovine placenta due to this “mad cow disease.” Shortly after Kojic acid was introduced as a QD in 1988. During that period this ingredient fell into disrepute due to alleged safety issues concerning possible carcinogenic effects (not confirmed since then). In view of this setback, most companies were condemned or limited to use vitamin C derivatives, with tyrosinase inhibition as the targeted activity. At present, the cosmetic industry is looking for exclusive ingredients from green and sustainable sources (preferably plants) that have a novel mechanism for skin whitening. Thus, there is a significant opportunity for ingredient seekers and formulators of novel, safe skin-whitening products that tie into the exploding demand by consumers for green and sustainable resources.
4.2.4.2 WHAT IS A WHITENING QUASI-DRUG?
It is not possible to talk about skin whitening without explaining what is the meaning of a whitening quasi-drug in Japan. A quasi-drug (QD) refers to the food, cosmetic, and drug standard regulated by the Japanese Ministry of Health, Labor, and Welfare (MHLW) under the Pharmaceutical Affairs Law. JSQI (Japanese Standard of Quasi-Drug Ingredients) and QD JSQI are the official standards set by the Japanese government for ingredients to be incorporated into final QD products, and labeled QD to advertise that the product is a government-approved medicated quasi-drug. This QD status has become a powerful marketing tool for many Japanese and foreign firms in the Japanese market. Obtaining QD status requires time and monetary investment, thorough documentation, and rigorous testing of safety and efficacy, all of which is regulated and approved by the MHLW. The efficacy of a QD is typically milder than that of a pharmaceutical drug, but has been proven effective in a specific claim category as either an excipient (additive) or an active at a specific dosage.
For skin whitening, this category in Japan is created for functional actives that will prevent hyperpigmentation or improve hyperpigmentary disorders. Most of the time the whitening products target an enzyme called tyrosinase. Tyrosinase is an enzyme needed for the production of melanin.
The following is a list of whitening QD in Japan in order of approval (from earliest to most recent) and it includes a description of their mode of action:
Bovine placenta:
This is a QD active that has been very popular and long used along with the use of vitamin C and its derivatives. The main source of placental extract was bovine, but it could also come from porcine origin, which has been recently used to replace the bovine due to the BSE issue. BSE was thought to be transmitted to human beings. The form of this disease in humans is the Creutzfeldt Jakob Disease, which killed numerous humans in the UK. Hatae et al. (1) and Ito (2) proposed, in the Fragrance Journal of Japan, the mode of action of placental extract as being an increased cell turnover to remove the melanin from the upper layers of the skin, or the role of high concentrations of minerals and amino acids, to reduce melanin synthesis.
Vitamin C (Ascorbic acid) derivatives:
Vitamin C and its derivatives are by far the most popular skin-whitening ingredients in use in Japan. Ascorbic acid and its derivatives are antioxidants, and as such they can block, for example, the conversion of DOPA to DOPAquinone; and from DOPAquinone, the melanin synthesis pathways can be blocked since the reaction pathway diverges to produce either eumelanin or pheomelanin (3). Most vitamin C derivatives approved as QD can be used as such by all customers. Some companies have had to obtain regulatory approval for the benefit of others! Takeda obtained the QD status for magnesium-ascorbic acid 2-phosphate in 1988 (use level must be 3%), and Shiseido obtained the QD status for L-ascorbic 2-glucoside in 1994 (use level must be 2%). Much later on, Nikko chemical, a supplier, obtained the QD status for use of tetra-isopalmitoyl ascorbic acid in 2007 (use level must be 3%). This oil-soluble form of vitamin C is easier to use and prevents UV-induced skin pigmentation through its antioxidative properties (4). The first company to introduce a QD product featuring this form was Green Coop in the QD line Shion.
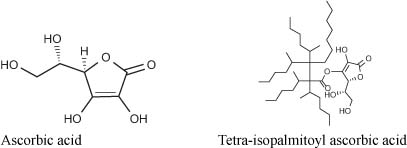
Other forms of vitamin C: vitamin C-ethyl, L-ascorbic acid2-phosphate, etc, . . . are on the market but not necessary as QD.
Kojic acid:
The hydroxylation of L-tyrosine to L-DOPA is catalyzed by the action of the copper-containing glycoprotein tyrosinase enzymes located in the melanocyte’s melanosome. Molecules chelating copper atoms can reduce the activity of the tyrosinase, and as a result, the melanin synthesis. This is how kojic acid functions as a skin whitener (5), and Shansho Seiyaku Co., Ltd obtained the whitening QD status in 1988.
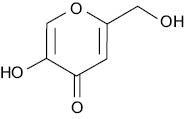
Kojic acid
Kojic is a QD at the use level of 1%; Mishima et al. evaluated the molecule in clinical tests to treat freckles, age spots, and post-inflammatory hyperpigmentation (6). Due to some concern about the potential carcinogenic effect, the MHLW (Ministry of Heath, Labour, and Welfare) started in March 2003 to discourage companies to use Kojic acid. Although they somewhat came back on their decision, most companies remain hesitant to resume its use and prefer to consider alternatives.
Arbutin:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








