Silicone Gel Breast Implants
Michael J. Miller
Breast implants are medical devices designed for surgical implantation to alter the size and shape of the breast in women. The principal indications are aesthetic enlargement and reconstruction of breast deformities related to cancer treatment, trauma, or congenital abnormalities. Breast implants are manufactured filled with either physiologic saline or silicone gel. Each has advantages and disadvantages, but silicone-filled devices are used most commonly and generally yield the most natural results.
Millions of women have had implantation surgery since the introduction of silicone gel–filled breast implants more than 40 years ago. It continues to be the most popular cosmetic procedure performed by plastic surgeons, with more than 300,000 performed annually (1). In the 1990s, sharp controversy arose regarding the health safety of silicon gel–filled breast implants, and their use was restricted by the U.S. Food and Drug Administration (FDA) in 1992. Ultimately no evidence was found to support significant safety concerns, and the devices were released again for general use in November 2006 (2). Nevertheless, some continue to question the safety of silicone gel breast implants, and FDA-mandated postapproval safety studies will be underway through 2018. It is possible that controversy will reemerge as data from these studies become available, and patients still need clarification of the safety issues involved.
For these reasons, it is important that plastic surgeons performing breast implant surgery understand the fundamental technology behind breast implants and be familiar with all the safety issues. Patients and others in their local communities will expect them to be experts. This chapter reviews the fundamentals of silicone chemistry, the history of breast implantation surgery and regulation, and current knowledge about safety and efficacy. The purpose is to equip the practitioner to hold a well-informed opinion about silicone gel breast implants, to be able to answer common questions about the use of breast implants, and to work with their patients to make sound clinical decisions about breast implantation surgery.
Silicone
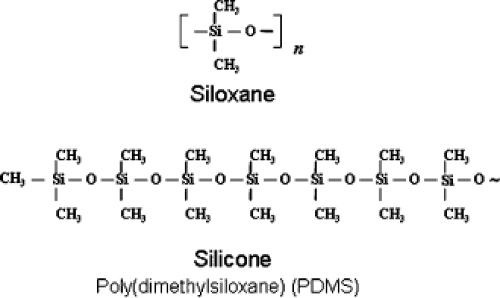
The fundamentals of silicone chemistry and nomenclature are summarized in Table 28.1. Silicone refers to family of compounds with a molecular backbone of alternating silicon (Si) and oxygen (O) atoms. Silicon is a semimetallic element found in nature as silica (SiO2), the most abundant substance on Earth, commonly found in nature in sand and quartz-containing rocks. Silicon is located just below carbon on the periodic table and therefore has a similar chemical behavior, most notably the ability to form long-chain molecules called polymers. The basic repeating unit (monomer) of silicone polymer is siloxane (R2SiO), named because it contains silicon, oxygen, and alkane (saturated hydrocarbon) side groups. The most common formulation used in medicine is poly(dimethylsiloxane) (PDMS), in which the siloxane monomer carries two methyl (—CH3) groups (Fig. 28.1).
Silicone polymers allow a high degree of control over the material properties of the final formulation, depending on the intended application. Modifying the length and molecular weight of PDMS polymers affects the mechanical properties. Low–molecular weight (<30 monomers) short chains yield materials with viscosity similar to baby oil. High–molecular weight formulations (≥3,000 monomers) are solids. Intermediate–molecular weight formulations can be designed to yield elastomers, nonliquid materials that can be deformed and return to their original shape without permanent alteration or destruction. Another factor used to control material properties is the degree of cross-linking between polymer chains. Cross-links are chemical bonds formed between long polymer chains, locking the long chains together and altering the properties of the final compound. Soft silicone is cured in a hydrosilation reaction in which some of the methyl groups (—CH3) on the siloxane backbone are replaced with vinyl groups (CH2=CH—), which can bond to receptive hydride groups (H–) in other chains to yield a lightly cross-linked elastomer with a compliance similar to human soft tissues.
The hydrosilation reaction is catalyzed by small amounts of platinum. Small amounts of residual platinum may be detected in the final product (3). Some forms of platinum have immunogenic potential, and the possibility of this as a source of adverse reactions to silicone gel breast implants has been suggested. However, no clinical reports have positively related the trace platinum found in breast implants to human disease, and the type and quantity of platinum used in manufacturing provide no biologically plausible rationale for health problems from this cause (4,5).
Silicone-based medical devices have been in use since the late 1950s (6). Excellent biocompatibility and versatile properties have led to diverse medical applications. Low–molecular weight formulations serve as lubricants for syringes needles, hand creams, facial cosmetics, food additives, and symptomatic treatments for gastrointestinal disorders. Higher–molecular weight versions are used to fabricate shunts and catheters, infusion ports, laryngeal implants, intraocular lenses, and coatings on the tissue interface of implantable devices such as cardiac pacemakers and implantable infusion pumps. Most medical devices implanted in soft tissues are coated with silicone at the material–tissue interface.
In plastic surgery, silicone devices have been used in a wide variety of clinical applications. Silicone implants are used to augment the craniofacial skeleton and reconstruct the orbital floor. They are used in hand surgery for joint reconstruction, flexor tendon replacement, and bone block spacers. Little controversy has surrounded the use of silicone for these applications. The application that has generated the most controversy is breast implantation surgery.
Table 28.1 Silicone Nomenclature | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
History
The modern history of surgery to modify breast appearance began in the late 1800s. Early techniques included autologous tissue transfers, but the complexities of tissue transfer led to attempts to implant a variety of synthetic and naturally occurring substances (Table 28.2). Direct injections initially became popular because of simplicity. Practitioners, often unlicensed, injected a variety of liquids and particulate suspensions directly into the breast parenchyma. This frequently resulted in a range of adverse outcomes up to and including complete loss of the breasts and death.
Medical-grade silicone appeared to be associated with the fewest complications and therefore held the most promise as an injectable material. In the early 1960s, the FDA approved a trial of liquid silicone injection for soft tissue augmentation. Nevertheless, up to 50% of patients with direct injection into the breast experienced adverse long-term results (7,8), and the practice has since been discontinued. Although the revival of direct injection of foreign substances is unlikely, injecting autologous fat processed from tissue aspirated during liposuction has been reported (9). Direct fat injection is associated with adverse results such as calcifications, cyst formation, localized induration, and abnormal breast cancer screening tests (10). Long-term results and best practices of these techniques are still under study, but they appear to have potential utility, particularly for localized deformities.
Difficulties associated with direct injection provided incentive to develop formed alloplastic devices for modifying breast volume. These required open surgery, but they afforded predictable and controlled localization of the material in the breast. In the early to mid-1900s, devices fashioned from a number of substances were tried, including ivory, glass, and synthetic polymers. Most led to contracting scar and a firm, unnatural appearance.
Table 28.2 Historical Materials and Devices Used for Breast Augmentation and Reconstruction | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Devices made of silicone polymers were a notable exception. Silicone had already demonstrated favorable performance in other medical applications such as urethral reconstruction, joint replacement, and implanted shunts. These successes led to the development of silicone gel breast implants. In 1963, Cronin and Gerow in Houston, Texas, reported the first use of silicone gel–filled breast implants (11). Over the next 25 years an estimated 1.27 million women received silicone gel–filled implants for either aesthetic breast surgery or reconstruction (12), and the number reached more than 2 million by the year 2000 (13). It is documented that currently more than 300,000 breast implantation procedures are performed each year in the United States (1).
Currently available breast implants are made of a silicone elastomer shell filled with either physiologic saline solution or silicone gel. Each has advantages and disadvantages. Saline-filled devices can be adjusted to optimum size at the time of implantation, but they can be unnaturally firm, form visible wrinkles on the breast surface, and move less naturally with changes in body position. Silicone gel–filled implants overcome these disadvantages because they simulate the density of natural human tissue. They are particularly useful when there is a small amount of overlying soft tissue as in augmentation of very small breasts or in breast reconstruction.
Implant Regulation
The timeline of key events regarding breast implant regulation is summarized in Table 28.3. Breast implants were introduced to clinical practice in 1963. The FDA did not regulate medical devices until 1976, when Congress enacted the Medical Devices Amendments to the Food, Drug, and Cosmetics Act (Public Law 94-295), placing all medical devices under regulatory control of the FDA. Silicone gel breast implants were introduced to clinical use prior to this and were considered “pre-amendments” devices. There are three categories of devices, depending on the level of risk for the patient and the degree of regulation thought necessary to ensure safety and efficacy (Table 28.4). Breast implants were initially considered class II devices:
intermediate risk with general controls and performance standards sufficient to assure safety and efficacy without special testing prior to release to market. During the 1980s, reports began to appear suggesting an association between medical silicones and systemic disorders such as cancer, connective tissue diseases, and other poorly characterized syndromes presumably related to adverse immune responses to silicone exposure. Coincident were clinical reports of patients being exposed to free silicone gel by diffusion across the elastomer shell (i.e., gel bleed) and implant rupture with uptake and transport of free silicone by the lymphatic system. Questions accumulated surrounding possible causal relationship between silicone exposure and a range of autoimmune disorders (e.g., systemic lupus erythematosus), arthritis, and collagen vascular diseases. Safety concerns were also raised related to adverse affects on the fetus and on infants during breast feeding by women with implants.
intermediate risk with general controls and performance standards sufficient to assure safety and efficacy without special testing prior to release to market. During the 1980s, reports began to appear suggesting an association between medical silicones and systemic disorders such as cancer, connective tissue diseases, and other poorly characterized syndromes presumably related to adverse immune responses to silicone exposure. Coincident were clinical reports of patients being exposed to free silicone gel by diffusion across the elastomer shell (i.e., gel bleed) and implant rupture with uptake and transport of free silicone by the lymphatic system. Questions accumulated surrounding possible causal relationship between silicone exposure and a range of autoimmune disorders (e.g., systemic lupus erythematosus), arthritis, and collagen vascular diseases. Safety concerns were also raised related to adverse affects on the fetus and on infants during breast feeding by women with implants.
|
Table 28.4 Device Classification by the U.S. Food and Drug Administration
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
|---|