Sclerotherapy of Small Veins
 INTRODUCTION
INTRODUCTION
Sclerotherapy is a useful adjunct to varicose vein ablation as well as a highly effective primary treatment for telangiectasias and associated reticular veins. Patients with axial varicosities often also have large numbers of small reticular veins and telangiectasias. Sclerotherapy presents a rapid, effective, and cosmetically acceptable treatment that is particularly attractive in patients with very extensive networks of small abnormal veins (Figure 13-1).
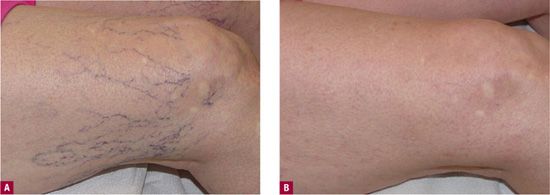
![]() FIGURE 13-1 Extensive network of protuberant telangiectatic webs, most efficiently treated by sclerotherapy A. Before treatment. B. After two sessions of sclerotherapy with 0.1 percent foamed sotradecol® in the feeder vein and 72 percent glycerin in the telangiectasia.
FIGURE 13-1 Extensive network of protuberant telangiectatic webs, most efficiently treated by sclerotherapy A. Before treatment. B. After two sessions of sclerotherapy with 0.1 percent foamed sotradecol® in the feeder vein and 72 percent glycerin in the telangiectasia.
Isolated small reticular veins and telangiectasias often cause severe symptoms that are worsened by prolonged standing or sitting, and that may be relieved by wearing support hose or by elevation of the legs.1 Vein size alone does not predict the presence of symptoms. Vessels causing symptoms may be as small as 1 mm in diameter or less.2 Besides symptoms of pain, burning, and fatigue, the appearance of the telangiectatic veins may be so disturbing that patients curtail their activities and modify their lifestyles to avoid situations in which their legs may be seen. Sclerotherapy not only offers the possibility of remarkably good cosmetic results but also has been reported to yield an 85 percent reduction in symptoms (Figures 13-2 and 13-3).3
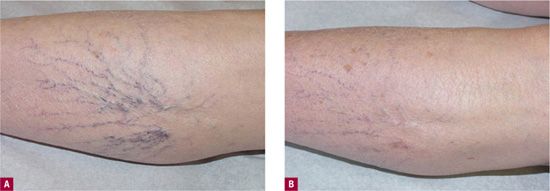
![]() FIGURE 13-2 Good cosmetic results and symptomatic improvement after sclerotherapy of painful telangiectasias. A. Before treatment. B. After three sessions using 0.1 percent sodium tetradecyl sulfate in the telangiectasias and 0.2 percent foamed sodium tetradecyl sulfate in the feeding reticular vein. Polidocanol 0.25% liquid for the telangiectasias and polidocanol 0.5% foam for the feeder could have been utilized as well.
FIGURE 13-2 Good cosmetic results and symptomatic improvement after sclerotherapy of painful telangiectasias. A. Before treatment. B. After three sessions using 0.1 percent sodium tetradecyl sulfate in the telangiectasias and 0.2 percent foamed sodium tetradecyl sulfate in the feeding reticular vein. Polidocanol 0.25% liquid for the telangiectasias and polidocanol 0.5% foam for the feeder could have been utilized as well.
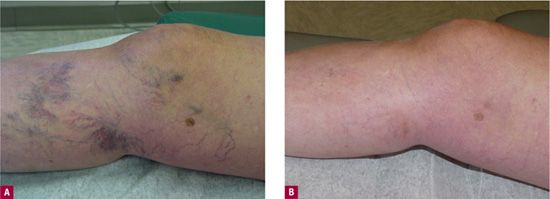
![]() FIGURE 13-3 Patient with painful residual telangiectasias after greater saphenous vein treatment. A. Before treatment. B. After three sessions with 72 percent glycerin.
FIGURE 13-3 Patient with painful residual telangiectasias after greater saphenous vein treatment. A. Before treatment. B. After three sessions with 72 percent glycerin.
Developing Expertise
A substantial investment of time and effort is required to develop expertise in sclerotherapy of veins of any size. Prior experience with venipuncture helps very little with treatment of larger veins and is completely irrelevant in the treatment of the smallest veins. Many physicians believe that expertise in venipuncture automatically confers expertise in sclerotherapy, which could not be further from the truth. Successful treatment requires the correct technique, diagnosis, and treatment plan for the type and size of vein to be treated. The procedure is mastered by reading written descriptions of proper technique and by observing and emulating the meticulous technique of skilled physicians. Many different techniques have been used in the treatment of small veins, but certain basic principles are universal.4–8
Classification and Anatomy
In order to diagnose and treat telangiectasias in a logical way, a classification scheme is helpful (Table 13-1). A discussion of painful telangiectasia includes telangiectasias (type I), venulectases (type II), and associated blue reticular veins (type III). For the purposes of sclerotherapy of telangiectasias, it will be assumed that the major primary varicose veins of types IV and V have already been eliminated or are absent.
TABLE 13-1
Classification of Veins35
Type I: Telangiectasia (spider veins): 0.1–1 mm diameter, usually red IA: telangiectatic matting (red network)
Type II: Venulectasia: 1–2 mm diameter, violaceous IIA: venulectatic matting (violaceous network)
Type III: Reticular varicosities (feeder veins): 2–4 mm diameter, cyanotic blue to blue green
Type IV: Varicosities (secondary saphenous branch or perforator related): 3–8 mm, blue to blue-green or colorless if deeper
Type V: Saphenous varicosities (truncal or axial varicosities including main saphenous trunks and first-generation branch varicosities): 5 mm or greater, blue to blue-green, colorless if deeper, may be palpable and not visible
TELANGIECTASIA FROM AXIAL REFLUX Telangiectatic webs may be the result of axial reflux due to junctional or large perforator incompetence.7 It is crucial to realize that axial reflux does not always result in large visible varicosities. Therefore, a detailed history and physical examination are essential to identify patients who require further noninvasive diagnostic evaluation prior to treatment. All large sources of reflux must be detected and eliminated before any treatment of telangiectasias. Failure to do so will cause a high rate of failures and complications, including telangiectatic matting, hyperpigmentation, and early recurrence.7
TELANGIECTASIAS FROM RETICULAR VEINS Telangiectasias can develop due to reflux from reticular veins, thin-walled, blue superficial venules that are part of an extensive network of subcuticular veins, otherwise referred to as the lateral venous system (LVS). When lateral, this network typically does not have direct connections to the saphenous system. Small perforating veins may be present particularly in the calf which connect to the deep venous system. Reticular veins associated with telangiectasias are commonly called “feeder” veins. With careful Doppler technique, free reflux with augmentation can be heard through these reticular veins, and ultra-high-resolution Duplex ultrasound has been used to map the path of transmission of venous hypertension from small reticular veins into telangiectasias (Figure 13-4).9,10

![]() FIGURE 13-4 Duplex ultrasound (10 MHz) showing reflux from reticular vein back to surface in the region of bridge of telangiectasias.
FIGURE 13-4 Duplex ultrasound (10 MHz) showing reflux from reticular vein back to surface in the region of bridge of telangiectasias.
ISOLATED ARBORIZING WEBS High-pressure reflux through failed valves is at the root of nearly all telangiectatic webs. When diagnostic tests fail to identify a large vessel or a reticular network as a source of reflux, localized small-vein valve failure usually is the culprit. Flow within even the smallest of venules is normally regulated by valves,11 and localized valve failure can produce arborizing networks of dilated cutaneous venules that are direct tributaries of underlying larger veins.12 Arborization occurs through a recruitment phenomenon in which high pressure causes dilatation of a venule, failure of its valves, and transmission of the high pressure across the failed valves into an adjacent vein. Treatment of an arborizing system must be directed at the entire system, because if the point source of reflux is not ablated, the web will rapidly recur.
 STEPS PRIOR TO TREATMENT
STEPS PRIOR TO TREATMENT
History and Physical Examination
No matter how small and how localized the telangiectasias, every patient must initially be evaluated by detailed history and physical examination, with noninvasive diagnostic vascular tests performed as necessary. Even when the physical examination is normal, historical factors may make further testing necessary. Previous venous surgery is an indication for more extensive evaluation, as is a family history of large varicose veins, since such patients are likely to have early axial reflux even when presenting with telangiectasias alone.13–15
Education and Consent
Once a diagnosis is made and the patient is judged to be a candidate for sclerotherapy, it is necessary to obtain informed consent. Photographs or a videotape is shown, and possible complications such as hyperpigmentation, matting, and ulceration are discussed. Patient education videos produced by the authors are available for viewing on youtube (e.g., http://www.mdlsv.com/Patient-Education, which includes three parts: causes and diagnosis of varicose and spider veins, methods of treatment, and side effects of treatment). The necessity for multiple treatment sessions is emphasized. If the treatment plan will include sclerosants that are used off label or are not FDA (Food and Drug Administration) approved, this fact must be clearly communicated. The solutions presently employed in the United States are FDA approved (see Chapter 14). After the patient understands the plan, risks, benefits, and alternatives, the consent form is signed.
PHOTOGRAPHS It is impossible to overemphasize the importance of photographs. As treatment progresses, patients invariably forget the original appearance of the treated areas. Pretreatment photographs are absolutely essential to help the physician evaluate treatment progress and to allow the patient to recognize improvement (see Chapter 25).
PRETREATMENT INSTRUCTIONS Patients are told to wear shorts and not to use moisturizers or shave their legs on the day of treatment. Shaving the leg may cause erythematous streaks, making it difficult to visualize patterns of reticular and telangiectatic veins. The use of moisturizers causes poor adhesion of tape used to secure compression following injections, but most importantly, it causes slower evaporation of alcohol used to prep the leg. Patients are encouraged to eat at least a small meal beforehand in order to minimize vasovagal reactions (see Chapter 25).
 FIRST TREATMENT “TEST”
FIRST TREATMENT “TEST”
The first treatment session is usually limited to a small number of sites in order to observe the patient for any allergic reactions, ability to tolerate the burning or cramping of a hypertonic solution, judge the effectiveness of a particular concentration and class of sclerosing agent, and observe any reactions to the tape or foam pads used for compression. It also serves to familiarize the patient with the treatment, treating physician, clinic surroundings, and the sensation of the fine needle. This allows more extensive treatment on the second visit, as the patient is typically more relaxed. The test site also complies with the suggestion in the package insert for sodium tetradecyl sulfate (STS). The IFU (instructions for use) or package insert for polidocanol (POL) (Asclera®) does not require a test-site injection.
When the patient returns in 2–6 weeks, the “test” site or limited treatment area is compared with pretreatment photographs. Any side effects such as matting and pigmentation can be explained to the patient. Reasonable time intervals for clearance of treated vessels can be reinforced. At each session, all sites treated are noted in anatomic diagrams in the chart.
 TREATMENT OF SMALL RETICULAR “FEEDER” VEINS
TREATMENT OF SMALL RETICULAR “FEEDER” VEINS
The same basic principle of treatment from the largest to the smallest varicosities holds true no matter what the size being treated. Sclerotherapy of telangiectasias begins with injection of the feeding reticular veins, then venulectases, then telangiectatic webs or networks, and finally the smallest and most isolated telangiectasias.
Treatment Plan
Continuous-wave Doppler or transillumination can be utilized when first beginning to treat reticular feeders and telangiectatic webs, because when a high point of reflux into a telangiectatic web can be identified, the injections are initiated at that point. When no clear feeder vessel is seen or identified by Doppler, injections begin at the point at which the telangiectasias begin to branch out. With increasing experience and recognition of common patterns, injection sites are based increasingly on known patterns of reflux, with treatment-session Doppler or Duplex ultrasound reserved for unusual cases and for patients who fail to respond to sclerotherapy. For example, reticular veins usually feed a group of telangiectasias on the lateral thigh from a varicose LVS. Similar common sites of reflux include lateral knee, posterior thigh perforating vein, lateral thigh perforating vein, and vein arising from the gluteal system. The treatment session would begin with reticular veins from which reflux is suspected to arise and would proceed along their course, with injections every 3–4 cm along the veins. It is neither possible nor advisable to treat every reticular vein of the thigh; only those reticular veins visibly connected to a telangiectatic web should be targeted. The “arrowhead” sign may be helpful in tracing the associated reticular vein back from a telangiectatic web (Figure 13-5).
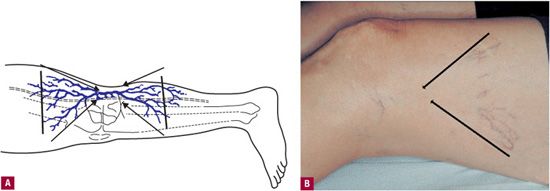
![]() FIGURE 13-5 Arrowhead sign. A. Schematic: When lines are drawn from the outermost telangiectasias of a telangiectatic web, they intersect over the reticular vein, which is connected to the telangiectatic group. B. Clinical example.
FIGURE 13-5 Arrowhead sign. A. Schematic: When lines are drawn from the outermost telangiectasias of a telangiectatic web, they intersect over the reticular vein, which is connected to the telangiectatic group. B. Clinical example.
As sclerosing solution flows away from the point of injection, it is diluted by blood and becomes less potent. This problem is addressed with foam sclerosants, which displace blood.
At some distance from the point of injection, the volume and concentration of the sclerosing solution will be insufficient to produce irreversible endothelial injury. When injecting a reticular vein, the sclerosing solution sometimes is seen flowing into the telangiectasias. When this happens, the telangiectasias often do not need to be injected directly. Similarly, the sclerosing solution injected into a telangiectasia may be seen flowing into the reticular veins, but these veins usually still need to be injected directly, as it is difficult to deliver an effective volume and concentration of the sclerosant to them indirectly.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


