5 Scalp and forehead reconstruction
Synopsis
 Anatomically the forehead and scalp have a complex three-dimensional anatomy that must be understood in order to perform reconstructive procedures successfully.
Anatomically the forehead and scalp have a complex three-dimensional anatomy that must be understood in order to perform reconstructive procedures successfully.
 The complex histology of the region allows for the development of a variety of unique congenital, traumatic, inflammatory, and neoplastic conditions.
The complex histology of the region allows for the development of a variety of unique congenital, traumatic, inflammatory, and neoplastic conditions.
 Reconstructive principles should be directed at replacing like tissue with similar tissue whenever possible. This is particularly important in the scalp given its unique hair-bearing characteristics.
Reconstructive principles should be directed at replacing like tissue with similar tissue whenever possible. This is particularly important in the scalp given its unique hair-bearing characteristics.
 Incisions in the scalp should be made parallel to the direction of the hair follicles with minimal electrocautery to minimize scars and alopecia.
Incisions in the scalp should be made parallel to the direction of the hair follicles with minimal electrocautery to minimize scars and alopecia.
 The aesthetic subunit principles of the face should be considered when cosmetically critical areas of the forehead are reconstructed in order to prevent a “patchwork” effect.
The aesthetic subunit principles of the face should be considered when cosmetically critical areas of the forehead are reconstructed in order to prevent a “patchwork” effect.
 Care must be taken not to displace mobile structures such as brows or eyelids when developing a reconstructive plan.
Care must be taken not to displace mobile structures such as brows or eyelids when developing a reconstructive plan.
 Reconstructive options include closure by secondary intention, vacuum-assisted closure (VAC) therapy, primary closure, tissue expansion, skin grafts, and a variety of local, regional, and distant flaps.
Reconstructive options include closure by secondary intention, vacuum-assisted closure (VAC) therapy, primary closure, tissue expansion, skin grafts, and a variety of local, regional, and distant flaps.
Historical perspective
Scalp reconstruction dates back to the Egyptians in 3000 bc.1 It evolved in response to traumatic injuries sustained in battle. Roman soldiers were known to have taken scalps as war trophies in approximately 100 bc.2 Scalping was a well-documented practice among native Americans, well before the arrival of Columbus.
During the Industrial Revolution, scalping was most commonly observed as a result of entanglement of hair in high-speed mechanical devices.3
Aulus Cornelius Celsus (c. 25 bc–50 ad) described in his book De Medicina the use of trephination of the exposed skull to allow the formation of granulation tissue with subsequent re-epithelialization.4 Scalp reconstruction has advanced from the application of split-thickness skin grafts in 1871 through replantation and free-flap reconstruction that we use today.5
Basic science/disease process
Anatomy
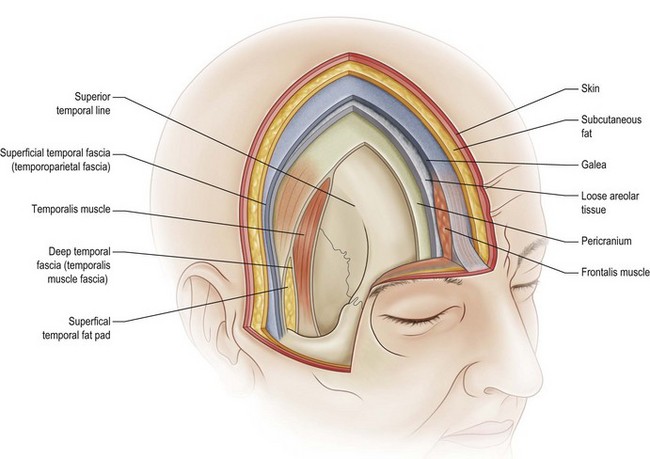
The scalp consists of five layers which can easily be remembered by the mnemonic “SCALP,” where S is skin, C is subcutaneous tissue, A is aponeurotic layer, L is loose areolar tissue, and P is pericranium (Fig. 5.1).6,7
Under the subcutaneous layer is the galea aponeurotica. This is a musculoaponeurotic layer that extends from the frontalis muscles anteriorly to the occipitalis muscle posteriorly. Laterally the galea continues as the temporoparietal fascia. This tissue is highly vascularized and has found great utility in reconstructive procedures about the head and neck. Anteriorly, the galea extends into the face as the superficial musculoaponeurotic system, as outlined by Mitz and Peyronie8 and later modified by Jost and Levet.9
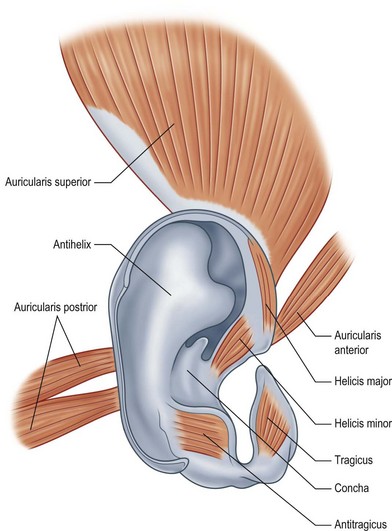
There are three auricular muscles on each side of the scalp: the anterior auricular, superior auricular, and posterior auricular muscles. They take origin from the temporalis fascia and the mastoid bone and insert into the perichondrium external ear (Fig. 5.2).
Anatomy of the temporal region
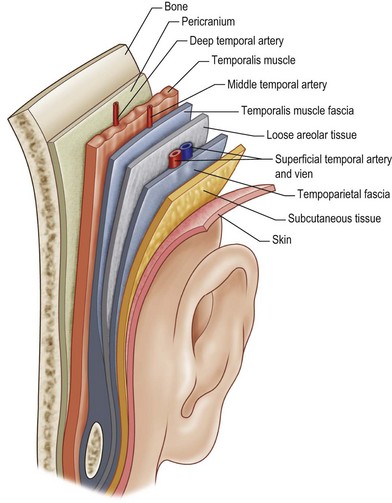
The temporoparietal region consists of four distinct fascial layers with anatomic significance. The most superficial layer is the superficial temporal fascia. This layer is a direct extension of the galea. It is closely applied to the overlying skin and subcutaneous tissue, making dissection difficult. Unless care is taken, it is easy to damage the overlying hair follicles, resulting in temporal alopecia (Fig. 5.3).
Blood supply
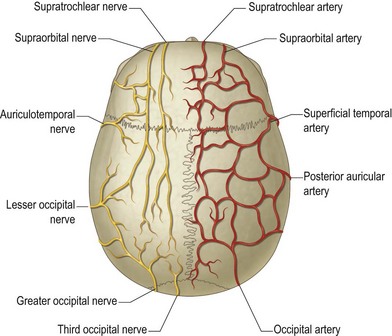
The scalp and forehead have a rich vascular plexus supplied by branches of both the internal and external carotid arteries. The supraorbital and supratrochlear arteries are terminal branches of the internal carotid arteries, providing the blood supply to the forehead and anterior scalp. The superficial temporal artery, posterior auricular, and occipital arteries are branches of the external carotid artery. These vessels supply the lateral and posterior aspects of the scalp. The extensive interconnection between each of the angiosomes allows replantation of the entire scalp based on a single donor vessel. The venous system parallels the arterial supply, eventually draining into the external and external jugular veins (Fig. 5.4).
Nerves
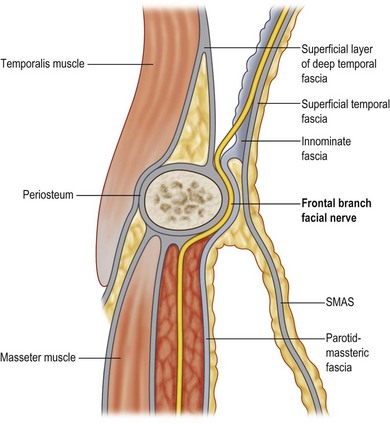
The frontal branch of the facial nerve supplies the motor innervation of the forehead. The nerve emerges from the parotid 2.5 cm anterior to the tragus. It ascends above the periosteum over the central portion of the zygomatic arch, passing 1.5 cm lateral to the orbital rim to innervate the frontalis muscles on their deep surfaces. It courses along the deep surface of the superficial temporal fascia, putting it at risk for injury with dissection into the temporal region (Fig. 5.5). The posterior auricular branch of the facial nerve supplies the occipitalis muscle. This branch originates with the facial nerve as it exits the stylomastoid foramen. The temporal muscles are supplied by the posterior and anterior deep temporal nerves, which are branches of the trigeminal nerve. This dichotomy of innervation is used successfully in developing reanimation procedures for patients with facial palsy.
The supratrochlear and supraorbital nerves, branches of the first division of the trigeminal nerve, provide sensation to the forehead and anterior scalp. The supratrochlear nerve exits the orbit between the pulley of the superior oblique and the supraorbital foramen. It ascends beneath the corrugator supercilii to supply the medial forehead, upper eyelid, and conjunctiva. The supraorbital nerve exits the frontal bone through the supraorbital foramen before dividing into two branches. The superficial branch of the supraorbital nerve courses over the surface of the frontalis muscle to provide sensation to the central forehead. The rest of the scalp and top of the head are innervated by the deep branch, which travels laterally between the periosteum and the galea. Knize10 has described the anatomy of the deep branch of the supraorbital nerve. The deep branch runs in a 1-cm-wide band medial to the palpable temporal crest line to innervate the frontoparietal scalp (Fig. 5.6).
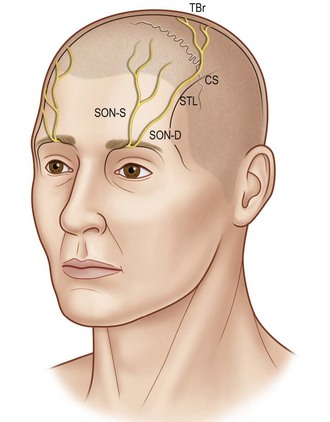
(Reproduced from Knize DM. A study of the supraorbital nerve. Plast Reconstr Surg. 1995;96:564–569.)
Aesthetic units of the scalp and forehead
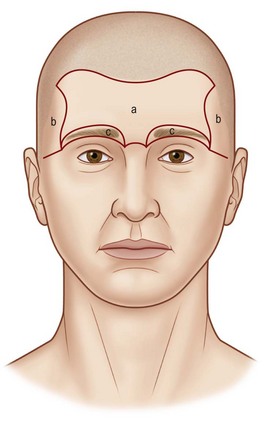
Gonzalez-Ulloa11 first conceived the idea of aesthetic units of the face. Facial aesthetic units and subunits are visual anatomic boundaries formed by contour changes in facial topography. Making incisions along these boundaries or replacing entire subunits hides scars in the light reflections and shadows of the face. The aesthetic subunits of the face must be respected when planning reconstructive procedures of the head and neck.
The upper third of the face historically has been subdivided into five units: two temporal units posterior to the anterior temporal crest, a central forehead unit (Fig. 5.7), and two eyebrow units along the supraorbital rims. More recently, the forehead has been subdivided into paramedian, lateral, and lateral temporal subunits.12 Care must be taken not to cause inadvertent displacement of mobile subunits such as the brow when operating on the forehead or scalp.
Hair structure and cycle
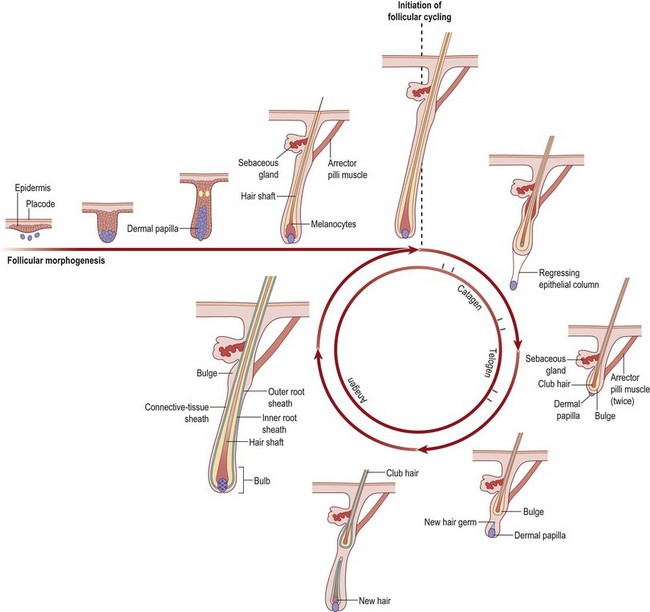
The follicle is an oval-shaped structure containing several different layers.12 At the base of the follicle is the papilla. It contains capillary loops that perfuse the growing hair follicle. Hair growth requires diffusion of oxygen and nutrients from the vascular network of the papilla. The hair bulb caps the papilla to form a bulbous expansion that forms the hair shaft.
The matrix of all hair follicles undergoes cycles of growth and degeneration (Fig. 5.8). The hair cycle has three stages of growth: anagen (growing phase), catagen (involutional phase), and telogen (dormant phase). At any one time, 90–95% of hairs are in the anagen phase, 5–10% are in catagen, and 1–2% is in telogen. The growing phase of human hair lasts about 1000 days. Catagen occurs for 2–3 weeks and telogen for 2–3 months. Up to 100 telogen hairs are lost from the scalp per day, an amount approximating the number of hairs entering anagen per day.
Disorders of the scalp and forehead
Cicatricial alopecia
Cicatricial alopecia is characterized by scarring of the scalp with resultant hair loss. It is caused by a number of pathologic conditions.13,14 The end result however is always the same: stem cell failure at the base of the follicle, inhibiting follicular recovery from the telogen phase. It can be classified into five categories: congenital, autoimmune, neoplastic, infective, and acquired. A complete discussion regarding each of these conditions is not within the remit of this article, Highlighted conditions of special interest to the practicing surgeon will be discussed (Table 5.1).
Table 5.1 Etiology of cicatricial alopecia
| Congenital |
| Neoplastic |
| Infective |
| Acquired |
Aplasia cutis congenita (ACC)
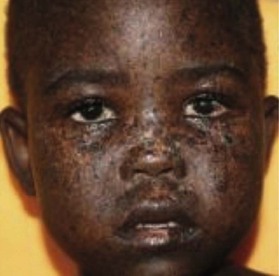
This condition was first described in 1176. Since then, more than 500 patients with this condition have been described in the literature. It is a rare congenital defect of the skin and subcutaneous tissue of the scalp. Less commonly, it can involve the periosteum, bone, and dura of the infant scalp.12,13 The scalp is the most common location for ACC. It is involved in 65% of all patients presenting with the disease. At birth these defects are usually covered with a thin fragile transparent membrane (Fig. 5.9). In older children, there is usually a hairless patch within the scalp resembling an atrophic scar. Less frequently, the lesions are found on the arms, knees, trunk, lower limbs, and face. Some patients with ACC also suffer from additional terminal transverse limb anomalies, nail hypoplasia, omphalocele, cardiovascular and central nervous system abnormalities.
Wound treatment in patients with superficial ulceration is generally conservative with regular dressing changes. Larger defects, especially with underlying bone defects, are susceptible to infection, meningitis, sagittal sinus thrombosis, and hemorrhage. In these deeper lesions, dural reconstruction, cranioplasty, and flap reconstruction may prove life-saving.15,16
Nevus sebaceous of Jadassohn (sebaceous nevus)
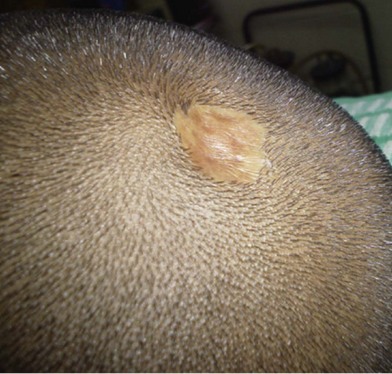
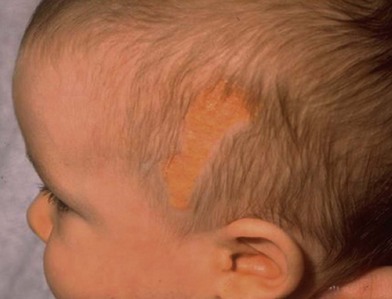
Jadassohn first described nevus sebaceum in 1895.17 It is a well-circumscribed yellow or orange lesion that occurs mainly on the face and scalp of infants. Of newborns, 0.3% are affected by nevus sebaceous. It occurs with equal frequency in males and females of all races. Clinically, the lesion presents as a solitary hairless patch noted at birth (Fig. 5.10). At puberty, they can become raised, thickened, and nodular.18
Histologically, it is a hamartomatous lesion consisting of predominantly sebaceous glands, abortive hair follicles, and ectopic apocrine glands. The entity is important to recognize, because of its propensity for malignant degeneration. Malignant transformation occurs in 10–15% of lesions in some series.19 The most common malignant neoplasm arising in this disorder is basal cell carcinoma. The most frequent benign tumor is trichoblastoma. Other benign and malignant tumors include syringocystadenoma papilliferum arising from the apocrine sweat glands, keratoacanthoma, apocrine cystadenoma, leiomyoma, and sebaceous cell carcinoma. Rarely, malignant eccrine poroma and apocrine carcinomas have been reported.
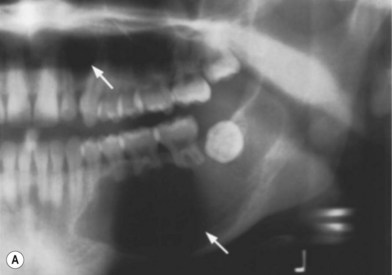
Nevoid basal cell carcinoma syndrome (NBCCS)
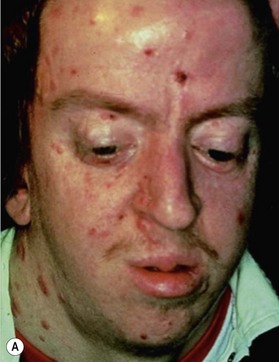
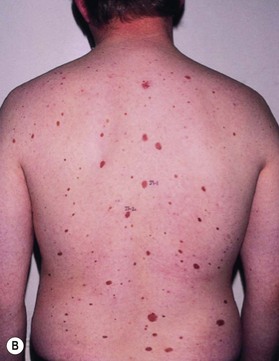
This is an autosomal-dominant condition associated with the development of multiple basal cell carcinomas of the skin (Fig. 5.11). First described by Gorlin and Goltz in 1960,20 it is an inherited disorder involving defects within multiple organ systems including the skin, skeletal system, endocrine and nervous system. To be diagnosed with the disorder, patients must meet two major criteria or have one major and two minor criteria (Table 5.2).21,22
Table 5.2 Nevoid basal cell carcinoma syndrome (NBCCS)
BCC, basal cell carcinoma.
Xeroderma pigmentosum (XP)
XP is an autosomal-recessive disorder characterized by intolerance of the skin to ultraviolet light. It has a prevalence of 1/250 000 in the US.23 Certain populations have a higher prevalence. For example, in Japan the prevalence is estimated at 1/40 000. The disease is due to the inability of the individuals affected with this disorder to repair damaged induced by sunlight to their DNA. Normally, damaged segments of DNA are excised and replaced with new sequences of bases. The most common defect in XP is an autosomal-recessive defect in which nucleotide excision repair (NER) enzymes are mutated, leading to a reduction in NER. Left unchecked, damage caused by ultraviolet light causes mutation in individual cell DNA. Seven XP repair genes, XPA through XPG, have been identified. These entities occur with varying frequencies, with XPA being the most common mutation. There is also an XP variant that has been described. The defect in this condition is not in NER, but is instead in postreplication repair. In XP variant, a mutation occurs in DNA polymerase (Fig. 5.12).
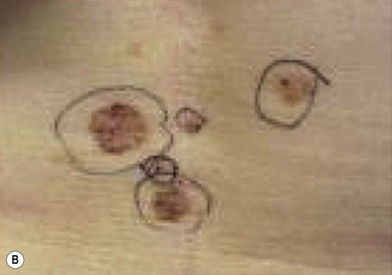
Giant hair nevus – congenital nevomelanocytic nevus (CNN)
CNN, commonly called the congenital hairy nevus, is a pigmented surface lesion present at birth.24,25 It is composed of neveomelancocytes, derivatives of melanoblasts. They are classified into three groups: small (<1.5 cm), medium (1.5–19.5 cm), and large (>20 cm in adolescents and adults or predicted to reach 20 cm by adulthood). CNN expands with growth of the child. The risk of melanoma development is proportional to the size of the congenital nevus (Fig. 5.13).
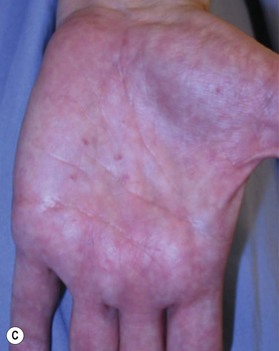
Dysplastic nevus
Dysplastic nevi are compound nevus with cellular and architectural dysplasia. They can be flat or raised and vary in size, but are typically larger than normal compound nevus (5–15 mm) with lack of pigment uniformity. Atypical moles may appear anywhere on the body, but most frequently occur on the scalp, chest, back, and buttocks. They may occur in sun-exposed and sun-protected areas. Atypical moles can be inherited or sporadic. The prevalence of atypical moles in white populations has been reported to be as high as 10%. Familial atypical moles may be inherited as an autosomal-dominant trait. This familial form of dysplastic nevi is known as familial atypical mole and melanoma syndrome (FAMMM) (Fig. 5.14).26,27
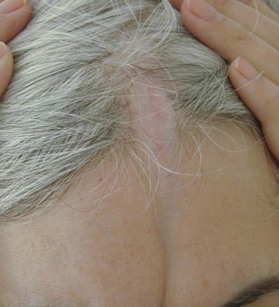
Linear scleroderma – en coup de sabre
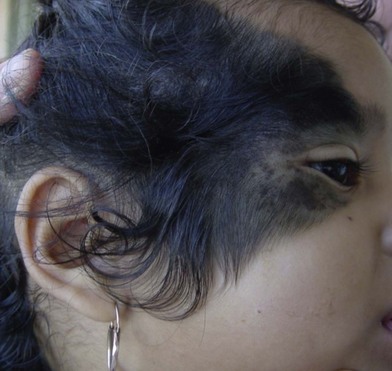
En coup de sabre is a form of localized linear scleroderma that primarily affects the forehead of affected pediatric patients. It appears as an indented, vertical, colorless line of skin. Its appearance to some resembles a deep saber wound. It is a rare disease of uncertain causation that is characterized by progressive craniofacial focal atrophy.28 The active stage usually lasts 3–5 years. Involutionary atrophy of skin, muscle, and even bone may occur. Various ophthalmological and neurologic abnormalities have been observed in patients with linear scleroderma en coup de sabre, including seizures and cranial nerve palsies. The distinction between linear scleroderma en coup de sabre and Parry–Romberg syndrome is unclear. Parry–Romberg syndrome is characterized by a gradual progressive facial hemiatrophy. In full-fledged cases, there is a significant deformity, with one side of the face smaller than the other. This is in sharp contrast to typical linear scleroderma en coup de sabre, where the abnormality is confined to the forehead (Fig. 5.15).
The management is unsatisfactory. Various therapeutic modalities (topical and pharmacologic agents, immunosuppression, and phototherapy) have been attempted, none with great success. Most require soft-tissue augmentation using microsurgical techniques once the condition has stabilized.29
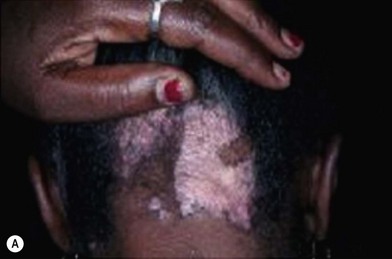
Discoid lupus erythematosus (DLE)
DLE is a chronic skin condition that appears as a red inflamed patch with a scaling and crusting appearance. The center may appear lighter in color with a rim darker than the normal skin. There is a predilection for the face, scalp, and ears; however other regions of the body can be affected. When lesions occur in hairy areas such as the beard or scalp, permanent scarring and hair loss can occur (Fig. 5.16).30,31 DLE may occur in patients with systemic lupus erythematosus (SLE) and some patients (<5%) with DLE progress to SLE.
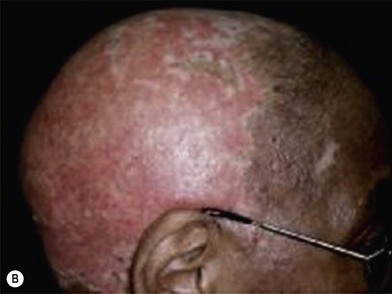
Cutaneous sarcoidosis
Lupus pernio is one of the few cutaneous manifestations that are characteristic of sarcoidosis. Lesions appear as indurated plaques that affect the midface, particularly the alar rim of the nose. Lesions of cutaneous sarcoidosis can also appear in pre-existing scars. This condition is called scar sarcoidosis. Therefore, sarcoidosis should be considered in the differential diagnosis of an enlarging, previously inactive scar. Often the lesion is mistaken for a keloid. Sarcoidosis of the scalp can result in scarring and nonscarring alopecia (Fig. 5.17). It is often mistaken for DLE, lichen planopilaris, and scleroderma. Local destruction and scarring of follicles in sarcoidosis may lead to permanent alopecia.32,33
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree