Fig. 20.1
Sprays have almost no sinus distribution to the paranasal sinuses prior to surgery. Mean sinus dispersion of radiographic contrast is extremely limited without surgical exposure of the sinus mucosa. This is particularly true of the frontal and sphenoid sinuses (Reprinted by permission of SAGE Publications, Harvey et al. [15])
Heterogeneity within published series in relation to the extent of surgery performed has made comparison of outcomes in interventions utilizing topical therapies difficult to assess, as some institutions will create widely connected cavities whereas others favor balloon dilatation or more conservative sinusotomies. Current evidence supports larger ostial remodeling, thereby permitting more effective delivery of topical therapies. A minimal size for effective penetration to the maxillary sinus is described as at least 4–5 mm [16–18]. For frontal and sphenoid sinuses, their more remote locations result in sinus surgery greatly influencing delivery of irrigation. More extensive surgeries such as medial maxillectomy and endoscopic Lothrop procedures further increase topical administration by creating a large, common cavity primed for success through effective penetration, improved topicalization, and easier long-term surveillance [11]. There is also clinical evidence from published RCTs that surgery improves the effects of topical therapies. A recent systematic review demonstrated the improved clinical impact of intranasal corticosteroid when used in previously operated patients compared to unoperated patients [19] (see Fig. 20.2).
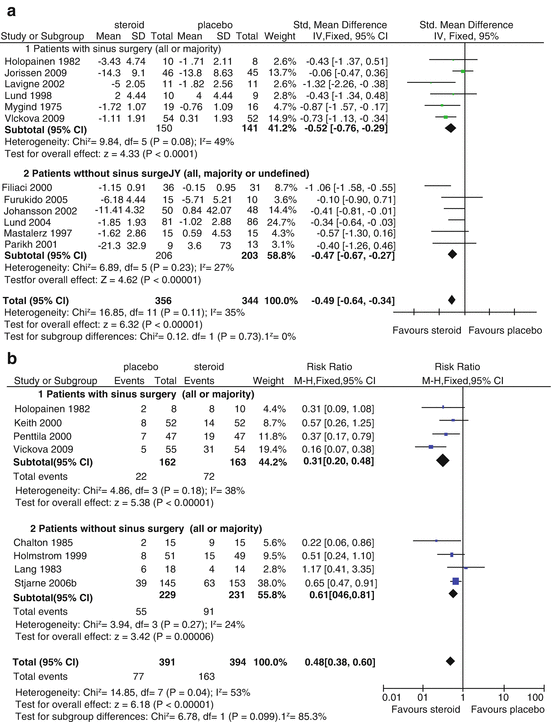

Fig. 20.2
Forest plot from meta-analysis of RCTs demonstrating benefit of nasal steroids versus placebo in patients undergoing sinus surgery from CRS. A meta-analysis demonstrating that the effects of INCS in the treatment of CRS are greater when looking at a group of randomized controlled trials if the studies are sub-analyzed by prior sinus surgery. (a) The standardized mean difference for symptom scores is greater in the studies that used groups of patients that have had prior sinus surgery, and the difference for overall “responders” to INCS therapy (b) is “significant” (Reprinted with permission from Snidvongs et al. [19])
Delivery Device
Research demonstrates significant variation in the distribution of different delivery devices to the sinuses. These devices can be divided into low- and high-volume delivery. Low-volume devices are typically common rhinitis medications which vary from 0.1 mL for a “spray” to 1–5 mL delivered by drops, atomizers, or nebulizers. A large volume device ranges from 60 mL to the more common 240 mL container and includes squeeze bottles, neti pots, powered irrigation devices, and bulb syringes.
Although sprays and drops have been traditionally used, they have fallen out of favor as an effective sinus delivery device. Objective measures of nasal spray devices have demonstrated that less than 1 % of the spray delivered to the nasal cavity reaches the paranasal sinuses [20]. Even with an optimal angle of delivery, nasal sprays are limited to the inferior and middle turbinate [21]. In the operated patient, nasal sprays fail to reliably reach the middle meatus least the paranasal sinuses, thus limiting their useful application in the pre- or postoperative state of the CRS patient [22]. While drops have been demonstrated to reach the olfactory cleft in a dependent position, overall, they are equivalent to nasal sprays in their limited distribution.
Nebulizers demonstrate limited delivery to the paranasal sinuses even in the postoperative state. There remains a lack of agreement in the literature in relation to the optimal particle size varying from small particles (<5 um) to larger particles [23]. Overall, nebulizers are good at moisturizing the nasal cavity but, like the other low-volume devices, fail to effectively penetrate the paranasal sinuses, even in the operated patient. Studies comparing the various low-volume devices have failed to demonstrate clear superiority of one over the others [12, 24]. The low volume of specialized pulsing nebulizers that might provide improved sinus penetration only works by delivering the fluid, and the mechanical action of clearing mucus is lost.
There are a range of high-volume devices; however when the volume delivered is >100 mL, the effective delivery to the paranasal sinuses is far more reliable. The sinuses represent a significant “dead space” beyond the nasal cavity, and a 100 mL + volume has been shown to most effectively penetrate these cavities [25].
Squeeze bottles, passive-flow devices such as neti pots, and pulsed irrigators all result in improved sinonasal distribution compared to low-volume devices. Clinical studies have also demonstrated improved patient symptoms and endoscopic appearance in postoperative patients with mild CRS [26], though in the same study, there was no improvement in the same outcomes versus sinus debridement alone for those patients with moderate to severe CRS. Pynnonen et al. [27] compared high-volume (240 mL) low-pressure isotonic irrigation to low-volume nasal spray and evaluated QOL and symptom scores in the postoperative period. They demonstrated improved QOL in both groups at 8 weeks but a significant improvement in both QOL and symptoms in the high-volume irrigation group.
Fluid dynamics between the different high-volume devices is likely to be very different. The high-pressure devices generate greater shearing forces on the mucous blanket; however, this is something that is very difficult to quantify at the research level (see Fig. 20.3 ).
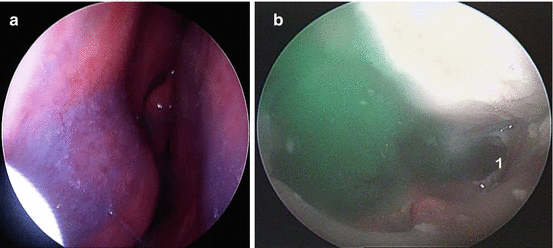

Fig. 20.3
Image (a) demonstrating the limited penetration of intranasal sprays with the nozzle of the nasal spray (a) seen in the nasal vestibule and the limited amount of methylene blue within the anterior nasal cavity. Even after endoscopic sinus surgery (ESS), the frontal recess (1) is not well penetrated with simple delivery. Only with head-down positioning and high-volume, positive-pressure irrigation does frontal sinus recess delivery occur (Image b)
Radiographic studies as well as endoscopic grading systems do not tend to distinguish between high-volume high-pressure devices, such as a squeeze bottle, and high-volume low-pressure devices, such as neti pots. It is not currently known whether high-pressure delivery offers an advantage over a high-volume low-pressure device. It has been demonstrated, however, that increasing the volume delivered results in improved sinonasal distribution [28, 29].
Position
A number of positions are available; these include the Mygind position (lying head back), the Ragan position (lying head lateral and low), and the kneeling position (head down and forward) (see Fig. 20.4 ).
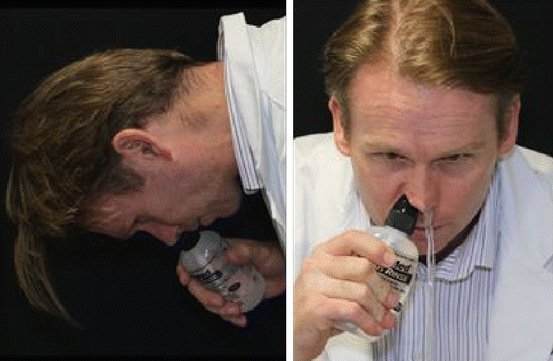

Fig. 20.4
The ideal position for maximal sinus penetration with an irrigation bottle is the “vertex down” position using sinus irrigation bottle (Model: A/professor Richard Harvey, Rhinology and skull base surgeon, Department of Otorhinolaryngology, St. Vincent’s hospital, Sydney)
Regardless of head position, sinus delivery is not reliably seen in the unoperated patient. For the operated patient, delivery to the paranasal sinuses is best seen in the kneeling position, though this is also found to be the most uncomfortable for many patients [11]. Well-designed studies seem to suggest that head position can affect sinus distribution when using positive-pressure devices such as a neti pot or bulb syringes. The lateral position appears more favorable for the neti pot [15], whereas the kneeling position results in improved sinus delivery (particularly to the frontal sinus) when using a “squeeze bottle” [25]. Most of the studies in position have used low-volume sprays and drops, and it is highly likely that a large volume mostly overcomes an advantage of positioning.
An important consideration is that in the elderly or the physically impaired, achieving these positions becomes increasingly challenging. In such a circumstance, a “head-over-sink” position may be preferable.
Based on the best available research, high-volume, positive-pressure irrigation devices appear to be the most effective delivery modality for penetrating the paranasal sinuses and overcome head position. In addition, both patients and prescribers require appropriate education on optimal volume and positioning based on the device used and the underlying mobility and dexterity of the patient.
Factors Influencing the Microenvironment
Mucous Blanket and Rheology
Nasal mucus is an important part of the body’s innate protection and represents the first line of defense to inspired particles. Up to 25 million particles are managed by the airway epithelium every hour with more than 500 l of air filtered in the same time period [30, 31]. The mucous blanket is a non-Newtonian shearing blanket that is composed of a watery periciliary layer and a viscoelastic gel outer layer. The thickness of these layers can be challenging to measure accurately; the inner layer in which the cilia reside is estimated to be about 5–10 um and the outer layer approximately 7–30 um in healthy mucosa [32, 33]. This can increase by as much as ten times in the diseased state thereby affecting mucociliary function as well as limiting diffusion of medications [34, 35].
This gel blanket that covers the respiratory epithelium is highly inducible and can increase in thickness by as much as 10 um per second when stimulated [36]. Hypersecretion of nasal mucus is a feature of CRS [37] as well as rhinitis [38] and is a recognized element in other inflammatory airway conditions. The implied role of sprays and irrigations is to remove particulate matter and reduce the load of antigenic or inflammatory debris; however, there is only limited evidence to substantiate this claim. There does exist indirect evidence in allergic rhinitis of reduced antigen-specific IgE in allergy sufferers who use saline during the allergy season [39].
Mucociliary clearance is comprised of a number of factors including ciliary beat frequency (CBF), ciliary structure, ciliary orientation, gel and sol composition, and mucus rheology. Studies have failed to demonstrate direct evidence of improved CBF with the topical delivery of saline; in fact, there is evidence of ciliostasis with hypertonic solutions at 7 and 14 % and reduced CBF with isotonic saline by as much as 46 % [40].
The effect of differing tonicities on mucociliary clearance and its effect on mucus rheology have also been explored. A blinded controlled study [41] (albeit in normal healthy adult patients) comparing buffered isotonic against buffered hypertonic saline demonstrated improved saccharine clearance time (SCT) for both. This reduction has been purported to be secondary to rehydration of the sol layer and better viscoelastic properties as a result of the increased ionic load, thus leading to more efficient movement of the cilia within the mucous blanket [42].
Effect on the Nasal Mucosa
Traditionally, it has been posited that saline irrigations are protective to the sinonasal mucosa by reducing mucosal dryness and by facilitating the clearance of thickened secretions and crusts. It has been further proposed that this could lead to improved IgA-mediated innate mucosal defense [43]. In vitro studies however have demonstrated morphologic changes in previously healthy mucosa with both hypo- (0.3 %) and hypertonic (3 %) saline solutions [44]. In the 0.3 % saline treatment group, normal human nasal epithelial (NHNE) cells were moderately damaged, and the total number of cilia-containing cells was also significantly decreased. In the 0.9 % saline treatment group, the epithelium appeared normal and was covered with healthy cilia. Cell-to-cell integrity also appeared to be maintained. When cells were treated with 3 % saline, holes appeared in places where secretory cells exfoliated, but cell-to-cell integrity was maintained. No studies to date have demonstrated beneficial changes histologically from isotonic solutions.
Tonicity
While there is a wide variation in individual thresholds and tolerance, the potential exists for increasing pain, secretions, and vasodilation with increasing tonicity. A number of randomized controlled trials have been performed evaluating outcomes in relation to symptoms and quality of life (QOL) comparing isotonic and hypertonic preparations [45], hypertonic versus no treatment [46], and isotonic irrigation versus reflexology [1]. Hypertonic saline has certainly been shown to stimulate the nasal mucosa. Hyperosmolar challenges have led to an increase in nasal secretions in concert with increasing osmolarity. A significant increase in secretions is seen first at 3.6 % in healthy patients [47], but even with 2.7 % solutions, burning and discomfort are experienced. In the viral rhinosinusitis sufferer, hypertonic saline solutions have demonstrated greater burning and discomfort when compared with an isotonic preparation, 32 % versus 13 % (p < 0.05) [48].
Proposed Decongestant Effect of Hypertonic Solutions
While there has been much speculation relating to the potential decongestant effect of hypertonic saline, this has not been supported in the scientific literature. This potential decongestant effect has been proposed through the effect of an osmotic process leading to reduced edema thereby improving nasal patency [48]. No statistically significant difference has been demonstrated at commonly used concentrations (0.3 % versus 3 % saline preparations) on rhinometric studies [49], and decreased airspace measurements have been seen on rhinometry following hypertonic saline exposure [50].
Adverse Effects
Overall, the use of nasal saline irrigation is regarded as low risk as demonstrated in the Cochrane review [51]. Often overlooked side effects however include cost, preparation time, and delivery effort. Some patients will find the practice of irrigation uncomfortable due to burning sensation, Eustachian tube dysfunction, and nausea. Our current protocols recommend twice-daily or less frequency schedules; more frequent protocols are simply not practical nor is there adequate evidence to support it.
The first description of contamination was by Heatley et al. in 2001 [52]. While not the main focus of the paper, it was recognized by the authors that colonization with bacteria happened in up to 30 % of neti pots or bulb syringes within 2 weeks. This is reflective of subsequent research evaluating colonization varying from 20 to 100 % of devices. While an in vitro study by Williams et al. demonstrated bacterial colonization of bulb syringes in the absence of human contact [53], it is widely accepted that device contamination is a result of colonized or infected sinonasal cavities [54].
Overall, Staphylococcus aureus and Pseudomonas aeruginosa are the most commonly isolated bacteria from irrigation devices. Geographical differences have been noted, with Pseudomonas more commonly isolated in the North American studies and S. aureus found to predominate in Australia.
Only one study to date has compared contamination between devices. Heatley et al. [52] found no statistically significant difference in contamination rate between open neti pots and the closed bulb syringe systems, although there was a trend toward fewer infections in the neti pot group [53]. Williams et al. also assessed the effect of tonicity and buffering and their effect on bacterial load. Unbuffered isotonic saline resulted in the highest contamination rates, with increasing tonicity producing an almost protective effect likely as a result of the optimal pH conditions for the proliferation of microorganisms (particularly S. aureus and Pseudomonas), seen at neutral/slightly acidic conditions.
Keen et al. [55] in an attempt to document the most effective cleaning method examined the success rates of the five most commonly recommended cleaning methods: rinsing with cold water, boiling water, detergents, Milton’s antibacterial solution, and microwaving. Although contamination still occurred with all cleaning practices, rinsing with boiling water or Milton’s solution or microwaving for 1.5 min on high appeared to reduce the degree of contamination.
While most manufacturers recommend changing the irrigation device every 3 months, a recently published small survey reported that most patients continue to use them for much longer, with the median duration of bottle use as high as 12 months [56]. A number of studies have assessed the effect of duration of use on colonization. While it was not found to be a linear effect, in most cases contamination increased with the duration of use. Despite the clear evidence of device contamination, the clinical effect is unclear. At the present time, it is recommended that devices be changed every 3 months and be washed between uses with clear education for the user on the optimal methods for sterilization and storage to reduce the burden of contamination.
Clinical Applications
Rhinitis
There are several well-performed clinical studies with patient-reported outcomes to support saline therapy in rhinitis management. In 2012, ten studies were identified that assessed the intervention of saline in patients with allergic rhinitis defined by a positive patient history or skin tests (skin prick) or blood test [57–65]. All ten studies were prospective and controlled with randomization not performed in only one [58] of the ten studies. The saline intervention was compared to no treatment in six studies [58–62], to INCS in one [63], to cetirizine in one [64], and to oil drops in one [57] and directly comparing isotonic versus hypertonic in one [65]. Combined analysis of eight of these studies demonstrated that the nasal symptom score improvement ranged from 3.150 to 67.159 % [66]. Saline irrigation failed to result in symptom improvement in pregnant patients and in fact caused mild worsening [62]. However, when compared to the control group that did not use nasal irrigation, the symptom score and consumption of anti-histamines were significantly reduced. Only two studies [63, 67] used high-volume nasal irrigation and four used a spray [57–59, 64




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree