RF Endovenous Occlusion (Closure™ or ClosureFast™)
 INTRODUCTION
INTRODUCTION
The development of minimally invasive methods to heat, denature, and contract varicose veins has been a major achievement in vein treatment and medical science, which began at the start of the 21st century. Endovenous ablation has replaced stripping and ligation, procedures that were first described at the beginning of the 20th century. Over 100 years ago, the Mayo stripper, Babcock stripper device, and Keller device were introduced as surgical instruments for avulsing varicose veins through surgical incisions in the groin and several points along the leg. These strippers are basically wires with an acorn-shaped head that pleats up the vein as it avulses the vessel loose from its attachments. These devices are rarely in use today. The Keller device is an internal wire used to pull the vein through itself, and surprisingly is sometimes still utilized even well into the 21st century as a perforation-invagination (PIN) stripper.
Over the last decade, endovenous occlusion techniques gradually evolved to become accepted as the gold standard. These minimally invasive alternatives to saphenofemoral ligation and/or stripping became adopted as more and more data accumulated indicating safety, efficacy, and successful long-term results.1 The first method of endovenous ablation to replace ligation and stripping was radiofrequency (RF) mediated shrinkage of the vein wall. This replacement for stripping surgery was first developed by dermatologic surgeons, Robert A. Weiss and Mitchel P. Goldman. The initial experience in the United States was published in early 2002.2,3 Critical to success of this Closure™ technique (VNUS Medical Technologies, San Jose, CA) was the application of dermatologic surgical tumescent anesthesia. A detailed discussion of the original endovenous RF ablation technique was included in the first edition of this textbook, the first textbook chapter ever published on endovenous ablation. Most of the world’s literature, however, still attributes development of endovenous ablation to vascular surgeons or interventional radiologists, while many surgeons still cling to the 100-year-old technique of stripping as a gold standard.4
Endovenous RF Closure™ was introduced into clinical practice in Europe in 1998 and in the United States in 1999, cleared by the U.S. Food and Drug Administration (FDA) for marketing in the United States during March 1999. Since then, hundreds of thousands of procedures have been performed worldwide. RF energy is delivered through a specially designed endovenous electrode to accomplish controlled resistive heating of the vessel wall. This causes vein shrinkage or occlusion by contraction of venous wall collagen (VNUS division, Covidien, Mansfield, MA) to eliminate saphenous venous reflux. Although the concept of endovenous elimination of reflux is not new, previous approaches have relied on electrocoagulation of blood. This resulted in thrombus occluding the vein, with the potential for recanalization of thrombus being very high. The concept of application of RF directly to the tissue, rather than blood, has been effectively applied for ablation of abnormal conduction pathways for arrhythmias.5 This concept was conceived for treatment of varicose veins, as venous occlusion with RF by the mechanism of venous blood coagulation has been previously reported but was not very successful.6,7 Another technique that preceded the modern application of RF to vein walls is endovascular diathermic vessel occlusion, a technique in which a spider-shaped intravascular electrode produced venous occlusion by electrocoagulation with minimal perivascular damage.8 Other variations of the use of RF have since been discovered that were performed behind the “iron curtain” of the Soviet Union. They utilized grounded RF delivered through an olive-shaped device placed intravenously. These failed; since heating and denaturization of vein wall collagen occurred, veins were coagulated in an expanded state rather than contracted and closed as with current technology. Patients were under general anesthesia for the olive-shaped device treatments since tumescent anesthesia had not yet been invented by dermatologic surgeon, Dr. Jeffrey Klein.9
Animal Studies of RF Ablation
The initial Closure™ system was developed using an animal vein model. Percutaneous access to goat rear leg saphenous veins was obtained through a 5 French introducer sheath and an RF catheter was positioned in a segment of the saphenous vein under fluoroscopic guidance. RF was applied as the catheter was moved distally along segments of the vein causing immediate contraction and cessation of flow that was visualized under fluoroscopy (Figure 23-1). The electrodes maintained direct contact with the vein wall to maximize vein wall heating and minimize blood coagulation.
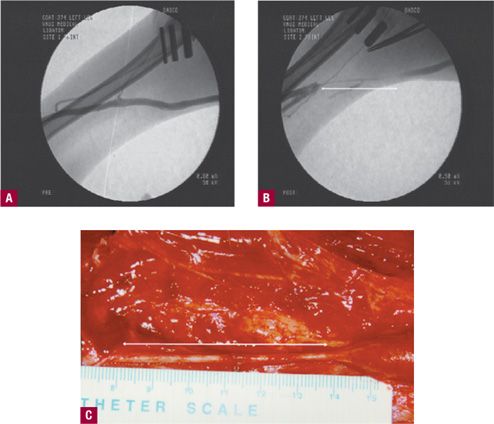
![]() FIGURE 23-1 Fluoroscopic guidance and direct visualization of goat saphenous vein before and after application of RF. A. Injection of radiopaque contrast before treatment. B. Immediately after treatment, no flow is seen after injection of contrast material in the segment shown by double-sided arrow. C. Similar segment seen intraoperatively six weeks after RF occlusion. Occluded fibrotic, cord-like segment is shown by double-sided arrow (Courtesy VNUS Medical).
FIGURE 23-1 Fluoroscopic guidance and direct visualization of goat saphenous vein before and after application of RF. A. Injection of radiopaque contrast before treatment. B. Immediately after treatment, no flow is seen after injection of contrast material in the segment shown by double-sided arrow. C. Similar segment seen intraoperatively six weeks after RF occlusion. Occluded fibrotic, cord-like segment is shown by double-sided arrow (Courtesy VNUS Medical).
Histologic changes confirmed the clinical findings in the animal studies. Acute histologic features included denudation of endothelium, some thrombus formation, thickened vessel walls, denaturation of tissue with loss of collagen birefringence, and neutrophil (polymorphonuclear neutrophil, PMN) inflammation. Depth of the vein wall damage was limited to 1–2 mm. Chronic histologic changes six weeks following RF occlusion demonstrated further reduction in lumen diameter to complete occlusion (Figure 23-2). A small residual lumen may be recognized but occluded by organized fibrous thrombi through the length of treated vein. Thrombus extension did not occur beyond the treatment site. Electron microscopic findings confirmed the light microscopic findings with marked endothelial damage and loss of the endothelium, neutrophils in vessel lumen, and thickened, bulbous collagen fibrils (Figure 23-3).
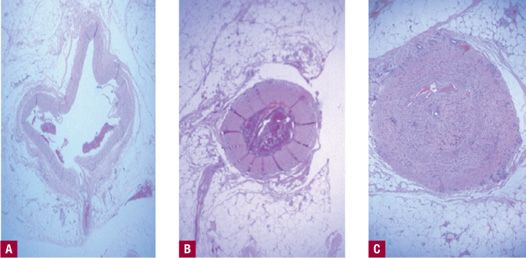
![]() FIGURE 23-2 Histology of RF occlusion. A. Before treatment. B. Acute histologic features of RF occlusion. C. At six weeks demonstrating fibrous cord with no recanalization (H&E 100X).
FIGURE 23-2 Histology of RF occlusion. A. Before treatment. B. Acute histologic features of RF occlusion. C. At six weeks demonstrating fibrous cord with no recanalization (H&E 100X).
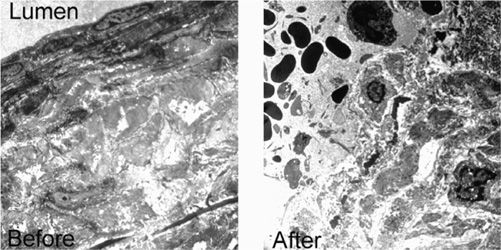
![]() FIGURE 23-3 Electron micrographs of histologic appearance before and immediately after RF occlusion of goat saphenous vein. Lumen is on the upper left. Note complete destruction of endothelium in the After photograph.
FIGURE 23-3 Electron micrographs of histologic appearance before and immediately after RF occlusion of goat saphenous vein. Lumen is on the upper left. Note complete destruction of endothelium in the After photograph.
From these histologic findings, it was concluded that acute contraction of myocytes and fibroblasts from thermal denaturation could be achieved by application of RF. This was accompanied by acute constriction and folding of intercellular matrix and collagen bundles. Abundant new collagen and intercellular matrix formation appeared within several weeks following RF occlusion. The result was a thickened vein wall with further constriction of lumen diameter. The potential safety of this technique was supported by the fact that in animal studies, there was no evidence of thrombus extension, while the zone of thermal damage had been limited to no more than 2 mm beyond the targeted vessel. A high acute success rate of 92 percent was followed by long-term vessel occlusion in the animal model.
 ORIGINAL CLOSURE™ TECHNOLOGY VERSUS CLOSUREFAST™
ORIGINAL CLOSURE™ TECHNOLOGY VERSUS CLOSUREFAST™
The current ClosureFast™ technology utilizes a method for vein heating that evolved from the original version (Figure 23-4). In the current iteration, directed RF energy that heats a wire coil at the energy-emitting end of an RF catheter transfers heat to the vein walls. The vein walls then heat shrink along with a denaturing of the vein wall protein. The previous mechanism by which RF current heated tissue was resistive (or ohmic) heating of a narrow rim (<1 mm) of tissue that was in direct contact with the electrode. This method was much more difficult to perform, as electrodes were susceptible to coagulum that would cut of the RF due to high resistance or impedance. The present system completely bypasses the problem with coagulum. Temperature, not ohmic resistance, is the limiting factor for the feedback loop.
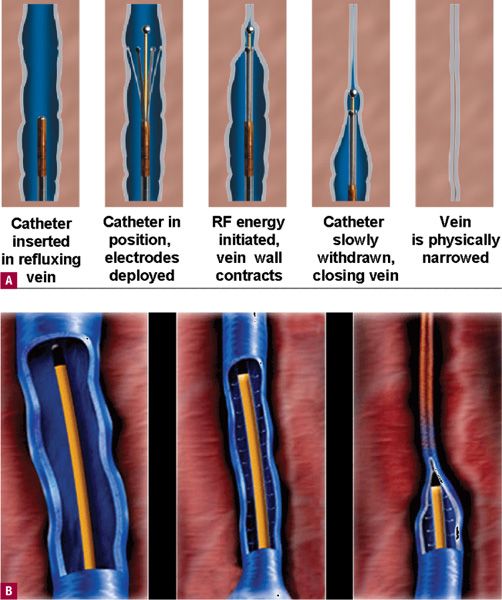
![]() FIGURE 23-4 Comparison of original design of RF catheter versus the new ClosureFast™ design. A. Electrodes contact the vein wall. B. A wire coil heats up using RF energy, which then secondarily heats the vein wall by direct contact as the catheter is pulled back (Courtesy of VNUS medical division of Covidien, Mansfield, MA).
FIGURE 23-4 Comparison of original design of RF catheter versus the new ClosureFast™ design. A. Electrodes contact the vein wall. B. A wire coil heats up using RF energy, which then secondarily heats the vein wall by direct contact as the catheter is pulled back (Courtesy of VNUS medical division of Covidien, Mansfield, MA).
It was thought that the optimal target temperature for vein closure™ was 70–90°C because tissue heated above 100°C begins to boil, but that is not the case as we now understand.10 All of the endovenous techniques heat the vessel at 120°C or beyond. Steam bubbles can reach much higher temperatures than water in the liquid phase. The temperature of steam can continue to rise well above 100°C if it is contained within a space, especially when additional heat energy continues to be added. These conditions are met with a continuously applied RF or laser within a confined space of a cylindrical vein.
ClosureFast™ replaced fast clogging electrode tips with a metal coil tip. A 7-cm long metal core at the tip of the catheter is heated to 120°F and allowed to transmit heat to the treated vessel for 20 s, which heat shrinks a vein 7 cm at a time to allow for very fast vein ablation. The ClosureFast™ catheter is shown in Figure 23-5. The simpler technique has sacrificed ability to treat segments shorter than 7 cm, with a rigid structure that limits treatment of more serpentine saphenous veins. In the original RF catheter system, the catheter was pulled through the vein while feedback-controlled with a thermocouple, while with the new system, the catheter is held in place while energy heats the catheter to a specified temperature of 120°C. This is believed to be a relatively safe process, as the temperature increase remains localized in a narrow rim around the active electrode. The RF device requires close, stable contact between the active electrode and the vessel wall and requires tumescent anesthesia to allow contact of the vein wall with the 7-cm metal coil. By controlling temperatures, boiling, vaporization, and carbonization of the tissues is avoided.11 With the previous generation RF system as shrinkage and compaction of tissue occurred, there was a decrease in impedance that decreased heat generation,12 but this is no longer the case as only the temperature of the catheter metal tip is monitored. Ten years of clinical experience suggest that the Closure™ procedure is highly effective at occluding saphenous veins and abolishing reflux. The efficacy of the newest iteration introduced in 2007 indicates that it is even more efficacious than the previous generation.13
![]() FIGURE 23-5 The ClosureFast catheter (Courtesy of VNUS medical division of Covidien, Mansfield, MA).
FIGURE 23-5 The ClosureFast catheter (Courtesy of VNUS medical division of Covidien, Mansfield, MA).
 CLINICAL EXPERIENCE AND SIDE EFFECTS
CLINICAL EXPERIENCE AND SIDE EFFECTS
Several studies have detailed the outcome of RF endovenous occlusion.2,14–18 In an initial study, there were 330 limbs of 294 patients in a prospective worldwide multicenter study with 31 participating sites and the patients were followed for three years post procedure.16 Varicose vein free rates were 90.1 percent at one year, 87.2 percent at two years, and 88.2 percent at three years. Duplex ultrasound demonstrated a reflux-free rate of about 88 percent over three years. Another long-term study of RF Closure™ of varicose veins followed the results of 1006 patients (1222 limbs) for five years. Vein occlusion rates were 87.2 percent at five years and reflux-free rates were 83.8 percent at five years.17 In our experience, the vein occlusion rates remain at 90 percent at over a decade of follow-up.
Side effects of the RF Closure™ procedure are typically minimal but are influenced by technique and anesthesia. Prior to the routine use of tumescent anesthesia, Sybrandy and Wittens from Rotterdam reported one-year follow-up of 26 patients treated with VNUS Closure™.18 They reported five patients with postoperative paresthesia of the saphenous nerve and one with a cutaneous burn for an overall complication rate of 23 percent. This illustrates the importance of tumescent anesthesia, as their use of a spinal anesthesia was clearly inferior. In addition, they treated patients from the ankle proximally, which exposed the great saphenous vein (GSV) within the calf to heat from the RF catheter. This is not recommended. Only the varicose portion of the GSV should be treated.
Another report describes two episodes of deep vein thrombosis (DVT) in 29 patients treated with the RF Closure™.19 The surgeons treated the patient with a groin incision and passage of the catheter from the groin downward. The authors do not report the type of anesthesia used or the length of vein treated. It is presumed that the patients were not ambulatory and treated under general anesthesia, which greatly increases the risks to the patients. Endovenous ablation should not be performed under general anesthesia.
 IMPORTANCE OF TUMESCENT ANESTHESIA
IMPORTANCE OF TUMESCENT ANESTHESIA
One of the greatest contributions to RF Closure™ endovenous ablation was the use of tumescent anesthesia. This application of tumescent anesthesia as part of the procedure of endovenous RF ablation was considered unique enough to be issued the following patents: US 6,258,084 B1 Method for Applying Energy to Biological Tissue Including the Use of Tumescent Tissue Compression and US 6,752,803 B2 Method and Apparatus for Applying Energy to Biological Tissue Including the Use of Tumescent Tissue Compression, which were issued to Mitchel P. Goldman, MD, and Robert A. Weiss, MD, among others. These patents were considered valueless at the time of issue but have resulted in awards of over millions of dollars during subsequent intellectual property litigation by VNUS medical against other manufacturers of endovenous ablation procedures, all of which require tumescent anesthesia.
We have maintained that the most important factor in minimizing side effects of endovenous RF ablation as well as other forms of energy for endovenous ablation is the use of tumescent anesthesia which allows for immediate ambulation. General anesthesia is the most significant risk factor in developing DVT and the failure to use tumescent local anesthesia is a major risk failure for developing skin burns. It is also advisable to limit RF treatment to the GSV segment above the knee to minimize the risk of paresthesia resulting from injury to the saphenous nerve. In our experience over a decade, using tumescent anesthesia with nonsedated patients, only two patients have developed focal numbness 4 cm in diameter on the lower medial leg. These were resolved within six months. No skin injury or DVT has been observed in any of our patients. Tumescent fluid compresses the vein, allowing intimate contact with the RF catheter. It provides excellent anesthesia and a heat sink for the rise in vein temperature to protect surrounding tissue. It should be applied using Duplex ultrasound guidance as described in more detail in Chapter 21.
Results of RF Endovenous Ablation
For clinical symptoms, the RF endovenous occlusion procedure rapidly reduces patient pain, fatigue, and aching correlating with a reduction in clinical severity, etiology, anatomy, pathophysiology (CEAP) clinical class for symptoms and clinical severity of disease (Table 23-1). When patients have had surgical stripping on the opposite leg, the degree of pain, tenderness, and bruising has been far greater on the leg treated by stripping. Skin burns occurred prior to the tumescent anesthesia technique in 2.5 percent, but this has not been seen since 1998. Other side effects, virtually eliminated by tumescent anesthesia, included clinical phlebitis at six weeks in 5.7 percent and temporary quarter-sized areas of paresthesia in 18 percent, with most of these occurring immediately above the knee and resolving within six months to a year. Endovenous RF occlusion has replaced traditional surgery of ligation and stripping of similar size saphenous veins. Most importantly for patients, side effects of DVT and nerve injury are far lower with RF when used with tumescent anesthesia compared to traditional ligation and stripping.
TABLE 23-1
CEAP Class Description with Findings after Endovenous RF Occlusion
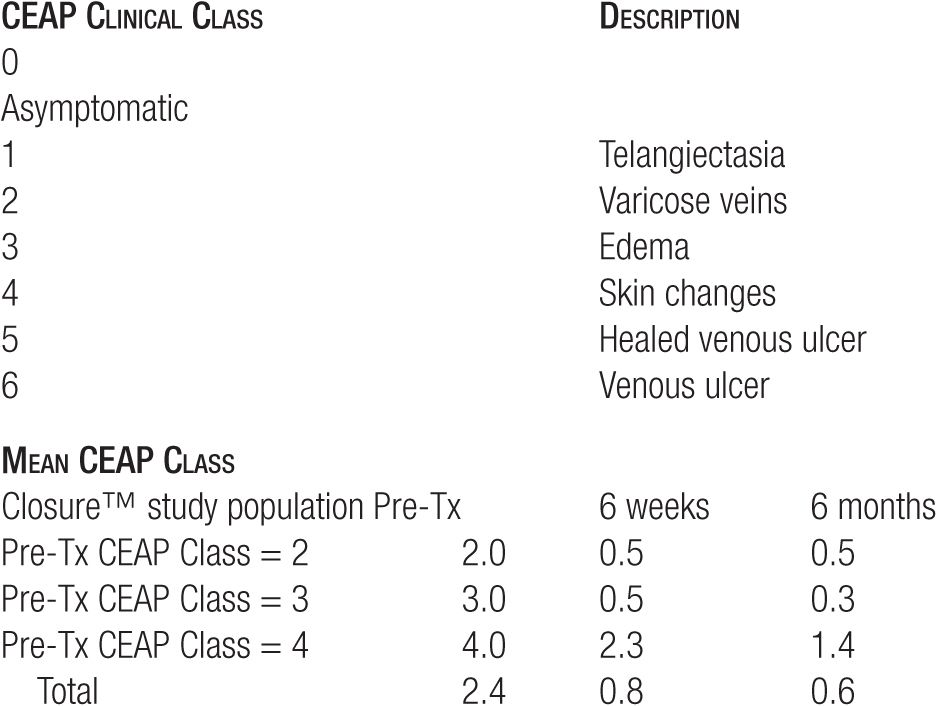
Our experience at 10 years indicates a 90 percent long-term effectiveness rate. This is a more favorable outcome on varicose veins than traditional surgery that has been reported to range in long-term efficacy at rates much lower than 90 percent.20 Patients remain symptom-free and patent GSVs are not visible under Duplex ultrasound. Clinical results observed at six months are indicative of the long-term results so that if the GSV is ablated at six months, it is likely to remain ablated at 10 years.
The superior epigastric vein that continues to empty superiorly into the common femoral vein is important to preserve and endovenous techniques are now performed so as to preserve this vein. Energy is applied starting at 2 cm below the saphenofemoral junction (SFJ) below the superior epigastic tributary. We have always believed that there is a high margin of safety by maintaining flow through this tributary. Among the many reasons, most important are that the high flow rate through the epigastric vein into the common femoral vein appears to diminish the possibility of extension of any thrombus (in the unlikely event that this would occur) from the GSV and normal venous drainage from the lower abdomen is allowed to continue. In our personal experience, thrombus has never been observed in over 1000 cases of endovenous ablation, although has been reported by others.19,20
 TECHNIQUE OF RADIOFREQUENCY CLOSURE™
TECHNIQUE OF RADIOFREQUENCY CLOSURE™
Patients with reflux in the great or small saphenous vein (SSV) are candidates. We have treated veins as large as 2.5 cm when standing, but which are compressed by tumescent anesthesia when the procedure is performed. A sample of the consent form is shown in Figure 23-6. Using Duplex ultrasound, the procedure begins with marking the vein to be treated on the skin. An appropriate entry point that is easily percutaneously cannulated is selected. This is usually just below where reflux is no longer seen in the GSV or SSV or where the vein becomes too small to be cannulated with a standard introducer set. For the majority of patients in our series, this is at a point just above or below the knee along the course of the GSV or just below the mid-calf with treatment of the SSV.
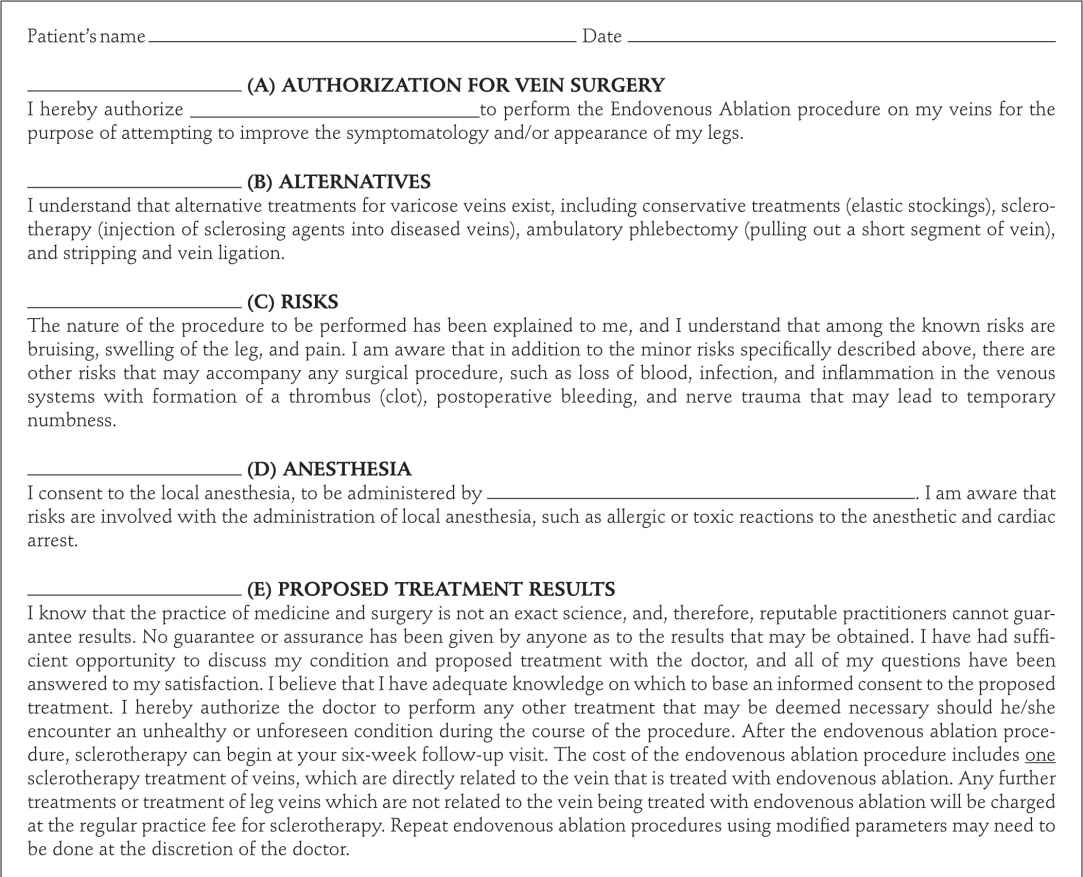
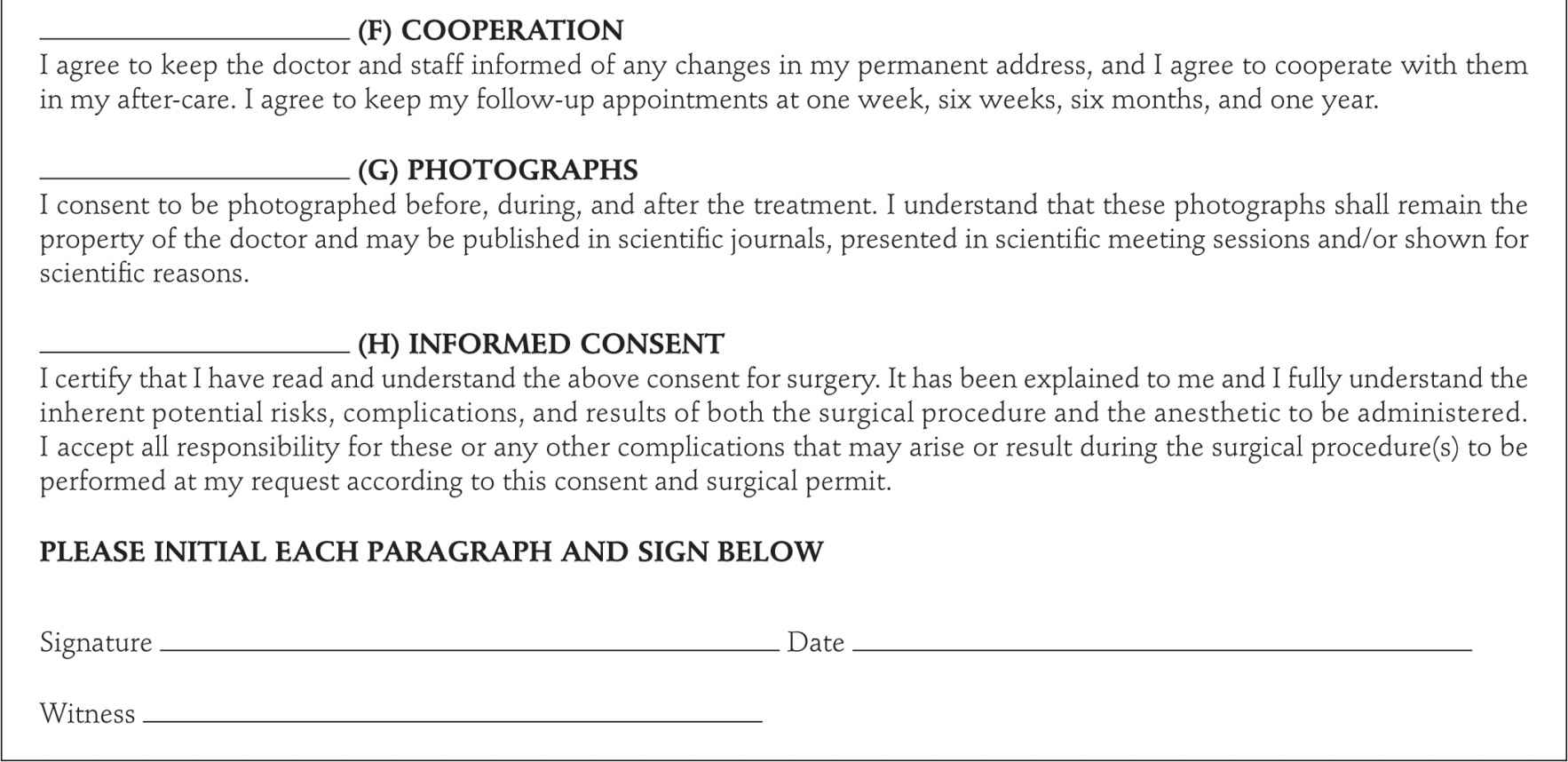
![]() FIGURE 23-6 Endovenous ablation consent form.
FIGURE 23-6 Endovenous ablation consent form.
Before proceeding, the patient’s feet are wrapped in thick socks to minimize vasoconstriction, a heating pad is placed under the thigh or around the foot, and a small amount of 2 percent Nitrol paste may be rubbed onto the intended entry point to minimize vasoconstriction during the initial cannulation process. The patient is then prepped and draped after which 0.2 cc of 1 percent lidocaine with or without epinephrine is injected at the ultrasound pre-marked site. It is important that the patient experiences no pain during cannulation, as the awareness or perception of pain will cause an immediate and rapid contraction of the saphenous vein. This makes cannulation almost impossible until the vein is no longer in spasm, which can take 30 min.
In order to place the sheath, a guidewire must be first inserted through the initial needle puncture. The guidewire is passed approximately 5 cm into the GSV. The sheath is then threaded along the guidewire, piercing the skin after a stab incision with a number 11 blade. Its progress is followed by Duplex until it is seen firmly placed within the lumen of the GSV. After establishing the intraluminal placement of the sheath, the guidewire is carefully withdrawn. Once the sealed sheath is placed into the vein, the Closure™ catheter is then advanced. Others prefer initializing entry via a venous cutdown or pulling of the vein close to the surface with an ambulatory phlebectomy hook. The current recommended technique is shown in the accompanying video and outlined by schematic and clinical sequence in Figure 23-7.
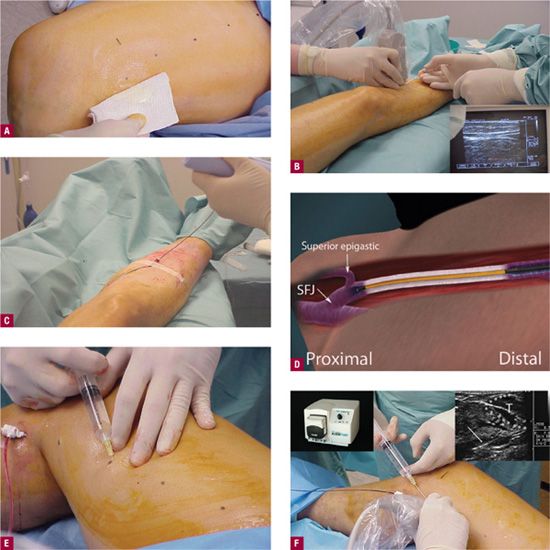
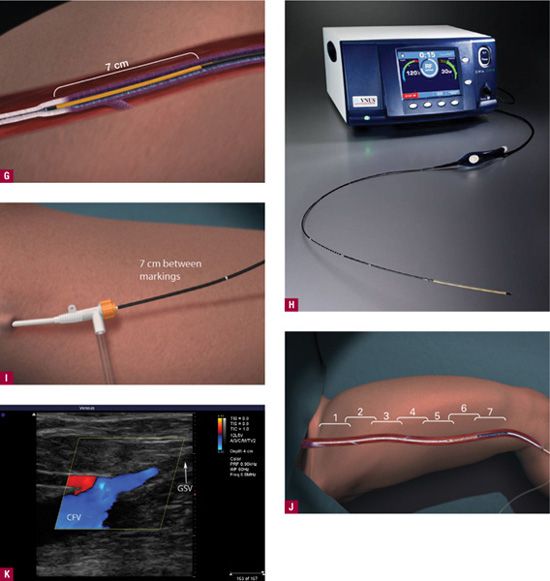
![]() FIGURE 23-7 Outline of procedure for ClosureFast™. A. Marking of the course of the GSV by duplex ultrasound with specific entry point noted by dots. Nitroglycerin paste is placed on the intended entry point to cause venous dilatation. B. Needle puncture guided by Duplex. Entry site monitored on Duplex screen showing appearance of 16-gauge needle or angiocatheter (inset). C. RF catheter is passed through access point. D. ClosureFast™ catheter advanced to 2 cm below the saphenofemoral junction. E. Local anesthesia is placed at the dots before tumescent anesthesia. F. Tumescent anesthesia is placed with tumescent anesthesia pump (upper left inset) instead of older syringe method shown. Tumescent anesthesia may be guided by Duplex to insure that anesthesia is being placed between the skin and the vein rather than below the vein. At least 2 cm of space is desirable between the treated vein and the skin to minimize risks to the skin. Upper right inset shows tumescent fluid (T) outlined by dots pushing down on the GSV. Catheter in vein is shown by arrow. G. A vein segment of 7 cm treated all at once during the 20-s treatment cycle. Additional vein segment treated serially. H. The RF console is monitored to insure heating to 120°C during the 20-s cycle. I. The catheter is pulled back by 7 cm for the next 20-s cycle. The catheter is marked with a white band every 7 cm for easy visualization at the access site. J. The vein is treated for its entire length in a stepwise fashion. K. As a result of the procedure, duplex ultrasound should show flow from the epigastric vein (red) into the common femoral vein (CFV), with occlusion of the GSV (arrow) up to the proximal 2 cm. This image is from four weeks after the procedure, as tumescent anesthesia prevents visualization of flow at the immediate conclusion.
FIGURE 23-7 Outline of procedure for ClosureFast™. A. Marking of the course of the GSV by duplex ultrasound with specific entry point noted by dots. Nitroglycerin paste is placed on the intended entry point to cause venous dilatation. B. Needle puncture guided by Duplex. Entry site monitored on Duplex screen showing appearance of 16-gauge needle or angiocatheter (inset). C. RF catheter is passed through access point. D. ClosureFast™ catheter advanced to 2 cm below the saphenofemoral junction. E. Local anesthesia is placed at the dots before tumescent anesthesia. F. Tumescent anesthesia is placed with tumescent anesthesia pump (upper left inset) instead of older syringe method shown. Tumescent anesthesia may be guided by Duplex to insure that anesthesia is being placed between the skin and the vein rather than below the vein. At least 2 cm of space is desirable between the treated vein and the skin to minimize risks to the skin. Upper right inset shows tumescent fluid (T) outlined by dots pushing down on the GSV. Catheter in vein is shown by arrow. G. A vein segment of 7 cm treated all at once during the 20-s treatment cycle. Additional vein segment treated serially. H. The RF console is monitored to insure heating to 120°C during the 20-s cycle. I. The catheter is pulled back by 7 cm for the next 20-s cycle. The catheter is marked with a white band every 7 cm for easy visualization at the access site. J. The vein is treated for its entire length in a stepwise fashion. K. As a result of the procedure, duplex ultrasound should show flow from the epigastric vein (red) into the common femoral vein (CFV), with occlusion of the GSV (arrow) up to the proximal 2 cm. This image is from four weeks after the procedure, as tumescent anesthesia prevents visualization of flow at the immediate conclusion.
With the old design of the Closure™ catheter, diluted heparin solution or normal saline was required to slowly drip through a central lumen, but the new design eliminates this concern. Passage of the RF catheter is monitored by duplex ultrasound. Once the RF catheter is in place, tumescent anesthesia is injected under duplex ultrasound guidance primarily between the superficial fascia, the cannulated GSV, and the deep fascia (the so-called SUPT technique for Subfascial Ultrasound-guided Perivenous Tumescent anesthesia). This is for the purpose of providing a heat sink, causing the vein to compress around the catheter and to provide a painless experience for the patient. Duplex monitoring of the anesthesia injection is not only recommended but is also essential to insure that the tumescent fluid is injected into the perivenous space and that the fascial planes are pushed away from the vein.
The final check of the position of the catheter is made with Duplex ultrasound. The tip of the metal core of the 7-cm long energy delivery tip is carefully placed 2 cm below the base of the terminal valve cusps. The readout of temperature prior to initiation of heating to 120°C should be substantially lower than body temperature due to the subfascial tumescent anesthesia. If the temperature readout from the thermocouple is higher than 28°C, then insufficient tumescent fluid has been injected. We prefer to see a baseline temperature after tumescent infiltration of 25°C–26°C. If the body temperature of 37°C is measured at baseline, some aspect of setup is critically wrong and the procedure should not continue.
The RF is then applied; the physician monitors the temperature and impedance. Within 10–15 s, the target temperature of 120°C should be reached. If this does not occur, the catheter has most likely been mistakenly advanced too far into the common femoral vein and the placement of the catheter needs to be carefully reassessed. After the target temperature is achieved, the catheter is moved 7 cm down using the markings on the catheter as a guide and the second application of energy occurs. It is important to prevent application of energy to the skin by making sure that markings on the catheter for the final 7 cm of application are carefully observed. At conclusion, duplex ultrasound of the SFJ should reveal no flow except for the superficial epigastric vein emptying into the common femoral vein. The GSV should be more echogenic with thicker appearing walls.
Comparison of RF to Endovenous Laser Ablation
Our experience is that postoperative pain is greatly reduced with the ClosureFast™ system compared with most laser systems that utilize wavelengths that target hemoglobin. These include wavelengths of 810, 940, 980, and 1064, but do not include 1320 and 1470 nm that target water. Several recent reports of patient experiences kept via a diary indicate less postoperative discomfort compared to the 980-nm wavelength.21,22 In this report, 81 patients returned a home diary (RF ablation = 45, 980-nm laser = 36). Patients receiving RF reported less postoperative pain than those receiving 980 nm at 3 and 10 days. Most significantly, those receiving RF via ClosureFast™ returned to work earlier than those receiving 980-nm endovenous ablation (median 5 versus 9 days).21 Clinical outcomes were similar at six weeks.22
When RF is compared to water targeting 1320-nm (CoolTouch endovenous ablation of varicose veins) endovenous laser, postoperative pain is virtually nonexistent with 1320 nm and most patients return back to work by the next day. Although these data are not yet published, this is our clinical experience using 1320 nm, which we have utilized for hundreds of patients since 2004. Clinical outcomes are excellent with both RF and 1320 nm, with successful saphenous occlusion in 90 percent of original RF, 98 percent of ClosureFast™, and 99 percent of 1320-nm treated GSVs.
Follow-up Care
Class 2 compression hosiery is worn for three days with the standard percutaneous Closure™ technique. Compression is worn for two weeks if sclerotherapy or ambulatory phlebectomy is performed on the same day. Anesthesia of the treated portion of the leg may persist for 8–24 hr with tumescent anesthesia. To gain experience, we recommend that for the initial cases, one should re-evaluate the treated veins at three days by duplex ultrasound. This will allow correlation of results with the pullback rate or any difficulty encountered during the procedure. Once comfortable with the procedure, the physician may want to see the patient for a duplex ultrasound follow-up study at six weeks. At that time, any open segments can be treated by Duplex-guided foam sclerotherapy. It has been our experience that when closed at six weeks, the GSV will remain closed, fibrosed, and almost indistinguishable from the surrounding tissue at six months in virtually all cases. Symptom reduction is rapid, with many patients experiencing relief at three days but some not until six weeks. Clinical improvement in appearance of varicosities is typically seen within six weeks as well (Figure 23-8). Although the patient instructions after the Closure™ technique are very straightforward including three days of compression, they are still provided with an instruction sheet (Figure 23-9). Ambulatory phlebectomy or sclerotherapy may be required as an adjunctive procedure to eliminate branch varicosities that do not resolve with closure™ or ablation of the GSV alone. This is to be anticipated, as many veins below the RF or laser endovenous ablation have been so overstretched for so long that smooth muscle cells have undergone apotosis. These veins are then no longer capable of contraction and remain like deflated balloons that need to be eradicated by sclerotherapy.
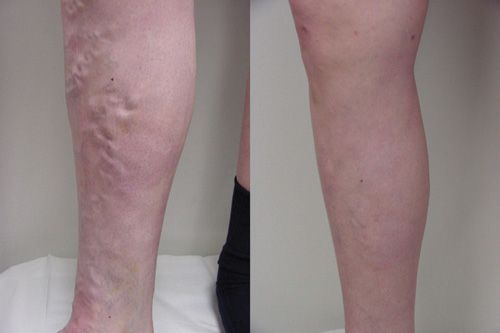
![]() FIGURE 23-8 Clinical improvement after RF endovenous ablation at six weeks after the procedure.
FIGURE 23-8 Clinical improvement after RF endovenous ablation at six weeks after the procedure.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


