Laparoscopic procedures for weight issues after primary LRYGB
Normal anatomy
Anatomical flaw (candy cane, pouch volume increase)
No weight loss → LSG
Bypass reconstruction (hyperphagia)
Weight regain → distalization (polyphagia)
→ Fobi ring (hyperphagia)
Laparoscopic procedures for weight issues after revisional or complicated LRYGB
Normal anatomy
Anatomical abnormality (GGF, marginal ulcer)
No weight loss → distalization
Bypass reconstruction
Weight regain → plication
Finally, an interesting option consists of laparoscopic reversal in which the initial anatomy is restored. Usually reversal will be complemented by an additional surgical action to reduce weight gain, and the procedure will consist of a sleeve gastrectomy (SG) with or without duodenal switch (DS).
Correction of Existing Flaws
With time, the gastric bypass construction may suffer unwanted anatomical changes. The pouch may dilate, and the GE can become too wide or, conversely, too narrow. In some cases, a gastrogastric fistula develops. Finally, a hiatal hernia, silently present preoperatively or acquired since the RYGB, may become symptomatic.
Pouch and Anastomotic Dilation
A pouch and/or a GE that is too wide impairs the restrictive aspect of the bypass and can contribute to weight problems. Treatment consists of reducing the size of the pouch and the anastomosis. This size reduction can be achieved surgically, by laparoscopically trimming the pouch and reducing the size of the GE (Fig. 27.1a).
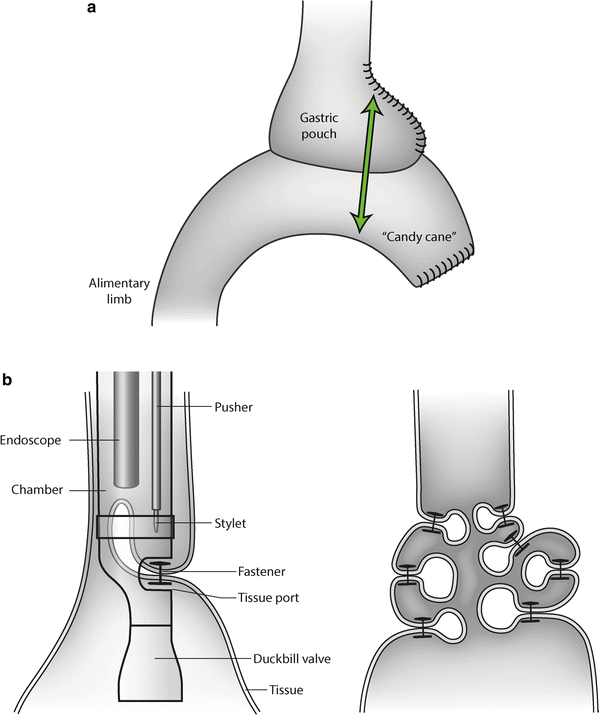

Fig. 27.1
(a) The dilated pouch, the widened gastroenterostomy, and the “candy cane” can be trimmed all at once by application of a linear stapler. (b) The StomaphyX™ (Endogastric Solutions, Redwood City, CA, USA) procedure for reducing compliance of the gastric pouch after gastric bypass. The “fasteners” induce permanent folds in the pouch wall
Recently, endoscopic attempts have been made to reduce the size and compliance of the gastric pouch and the GE by injecting substances such as morrhuate, as described by Dargent, or by endoscopically plicating the gastric wall at the level of the pouch. Plication can be achieved by firing fasteners as described with the StomaphyX™ device (Endogastric Solutions, Redwood City, CA, USA) (Fig. 27.1b).
The GE can be narrowed by endoscopic suturing as described with the ROSE [2] procedure. However, the literature reveals a lack of long-term positive outcomes.
Anastomotic Stenosis and Strictures
In cases in which the GE has become too narrow, such as after chronic ulceration at this level, or because of ischemia, pneumatic balloon dilation may be attempted. It is essential to use high dilation pressures and to maintain the dilation for a sufficiently long time as described by Galvao-Neto and colleagues [3]. Usually, a partially covered self-deploying metallic stent is inserted to maintain the dilation. Alternatively, the stricture is incised by an endoscopic cold or hot knife, but this technique has not yet been widely accepted [4].
In many cases, the stricture is caused by chronic peptic ulceration. Peptic anastomotic ulcers are notoriously therapy resistant and are a frequent cause of reoperation [5]. Surgical options include laparoscopic trimming of the gastric pouch, resection of the GE, and reanastomosis a few inches distal on the AL, but recent reports advocate the resection of the remnant together with the gastric pouch and the anastomosis and reanastomosis of the AL to the distal esophagus [6]. In the author’s experience, this latter technique was used a number of times in revisional bypass after adjustable band gastroplasty (LAGB). This technique was advantageous in terms of complications but was characterized by poor weight loss and was abandoned in our practice. The author prefers to perform a truncal vagotomy and a resection of the fundic part of the remnant to deal with Ghrelin production and to avoid gastrogastric fistulas. At the same time, the author resects the proximal part of the AL, the anastomosis, and the distal part of the gastric pouch to obtain a micropouch, as described by Sapala, to further reduce acid production in the gastric pouch.
Gastrogastric Fistula (GGF)
The gastric remnant may harbor a gastrogastric fistula, a condition caused by an incomplete division of the stomach at the time of the initial surgery or created by a perforating ulcer of the pouch, which results in a “de facto” abolition of the bypass. The symptoms are those of a “normal” situation: Weight loss usually halts and the patients often complain of significant GERD. In the case of a perforating ulcer, the patient usually recalls an episode of pain that suddenly stopped at the time of the actual perforation. In some cases, the ulcer addresses the anastomosis or the proximal part of the AL. The treatment usually consists of truncal vagotomy, resection of the lateral part of the gastric pouch, or, in cases of anastomotic or AL ulceration, resection of the distal part of the pouch and the proximal part of the AL, including the anastomosis, and always includes resection of the fundic part of the remnant. In the author’s experience, while extremely effective in terms of weight loss, this technique results in a high number of leaks [7].
To avoid fistulas, a number of attempts have been made to close the GGF endoscopically. Despite encouraging early results, up to 70 % of GGF recur within a year [8]. New devices seem promising, including the wolf-trap-like “over-the-scope” (OTSC) clip (Fig. 27.2a–c) [9], but long-term results are yet to be determined.
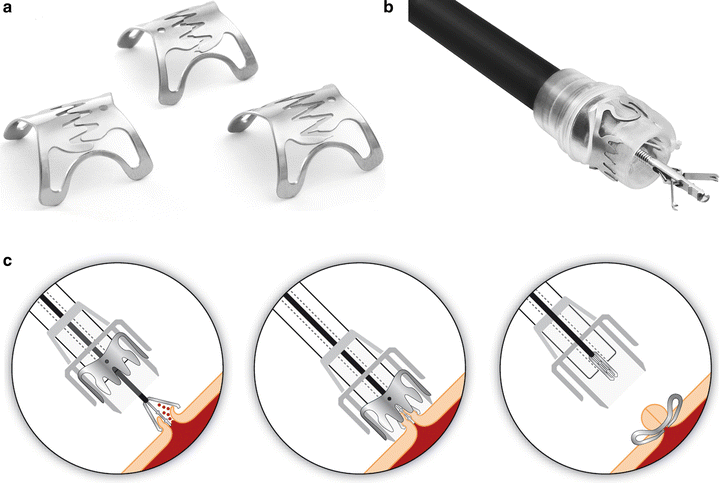

Fig. 27.2
(a, b) The wolf-trap-like over-the-scope (OTSC) clip by Ovesco (c) keeps the edges of the defect in close apposition (Reprinted by permission of Ovesco Endoscopy USA Inc., Los Gatos, CA, USA)
Hiatal Hernia and GERD
LRYGB is known to cure GERD. As a consequence, LRYGB is the corrective procedure of choice for patients who develop significant reflux after laparoscopic sleeve gastrectomy (LSG) [10]. However, GERD may complicate even the RYGB. Usually the reflux will be alkaline, but acid reflux is possible as well. With acid reflux, a gastrogastric fistula must be ruled out. Other possible causes of acid GERD include too short an AL and/or a downstream obstruction, at the level of the enteroenterostomy (EE) or further distally. In case of acid reflux with a long AL and in the absence of a problem at the EE itself, an incisional hernia should be suspected. In our experience, the hernia is typically located at the level of the left upper quadrant where we routinely place a 12 mm trocar to accommodate the stapling device.
When anatomical flaws of the bypass construction have been ruled out, GERD typically is concomitant with a hiatal hernia. A hiatal hernia after LRYGB may be highly symptomatic. In addition to the symptoms of reflux, patients may complain of vague discomfort in the left shoulder and the left side of the neck [11]. In some patients, the chest pain is reminiscent of heart problems. In exceptional cases, the hiatal hernia acts as a paraesophageal hernia because the gastric pouch remains attached distal to the diaphragm, but the AL moves inside the chest and directly irritates the heart mechanism. We have observed two cases of intrathoracic migration of the upper part of the remnant, including in one patient who developed incarceration and necrosis of the upper pole of the remnant. Due to these aforementioned dangerous conditions, we now routinely explore the hiatus in all patients we submit to LRYGB. This exploration includes a thorough search for a prehernial lipoma, which must always be reduced. In addition, we systematically re-explore the patients with a clinical suspicion of hiatal hernia at some point after the bypass. When correcting a hiatal hernia, we perform a thorough circumferential dissection of the crura and the distal esophagus. We also routinely perform hiatoplasty by placing posterior sutures to obtain an angulation at the esogastric junction, a condition that is essential in avoiding reflux. We have had poor results with the use of prosthetic material to reinforce the crural repair. In two of the five patients in whom we attempted this technique, we found intragastric erosion of the prosthetic material, although we exclusively used biomaterial (Surgisis®, Cook, Bloomington, Indiana, USA). Erosion of a reinforcing mesh into the small gastric pouch is best treated by resecting the pouch and constructing an eso-enterostomy [12].
Changing the Bypass Components to Influence Restriction and/or Malabsorption
One of the main problems with LRYGB is related to weight issues.
We found that one of the leading causes of insufficient weight loss or weight regain is the development of poor eating habits, more specifically sweets eating. This evolution is facilitated by poor follow-up. When inadequately monitored, bypass patients easily relapse into their old eating habits. Therefore, the first step in treatment is an adequate dietary interview to detect the dietary flaw. Thorough, continuous dietary counseling is next. Changing eating habits, however, remains a tremendous challenge in a bariatric population, and surgical treatment appears to be the only option in some instances.
When patients eat too frequently (polyphagia, grazing), additional malabsorption can be achieved by manipulating the limb components of the bypass. Less aggressive changes to the AL and CL have been shown to be futile in the long term [13].
Despite Sugerman’s catastrophic experience with distal bypass [14], we have followed Brolin’s philosophy [15] and, in some occasions, have transformed the regular LRYGB into a distal bypass. We transected the AL flush with the EE and reimplanted it approximately 150 cm proximal to the ileocecal valve [16]. Because the AL is usually approximately 150 cm in length, we thus ended up with a total of 300 cm of active bowel length, the minimum length to ensure adequate protein absorption, according to Scopinaro [17]. This construction is quite different from Brolin’s technique (Fig. 27.3), in which the CL is just 75 cm long [15].
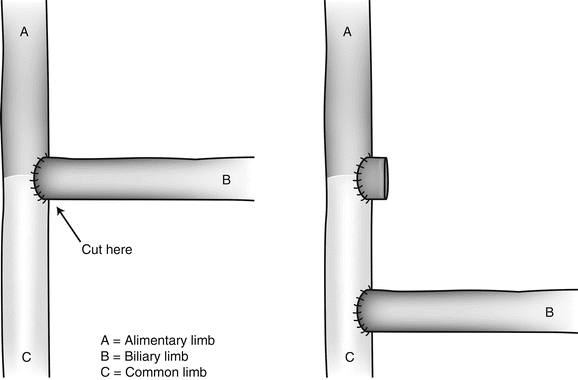

Fig. 27.3
In Brolin’s technique, the BL is reimplanted at some 75 cm proximal to the ileocecal valve. With this technique, the AL is extremely long
Most authors warn against the possible unwanted long-term outcomes of the technique. However, there is a substantial difference between a distal bypass as a primary attempt and a distal bypass as a remedial technique as we advocate here. In the latter case, patients no longer demonstrate substantial restriction, unlike in the primary cases where restriction is still significant. In addition, reserving distalization to remedial cases allows the surgeon to select patients who have demonstrated compliance during follow-up. An important condition of distal bypass is continuous, assiduous follow-up to determine the progression of plasma protein, minerals, and fat-soluble vitamin levels. In some cases, hypoproteinemia becomes a life-threatening condition and the only remaining treatment option is to reconvert the bypass to a normal anatomy. Reversal is a realistic option after laparoscopic RYGB, but there are recently published reports on reversal after open bypass as well. Despite the technical feasibility, we believe that straight-on reversal after distal bypass is dangerous because of an increased leak rate related to low protein content. To avoid this hazard, we prefer to perform a feeding gastrostomy, followed by tube feedings for a few months. Tube feedings are less expensive, less prone to infections, and are easier to implement practically than intravenous parenteral hyperalimentation. We usually prescribe tube feedings of full milk, a readily available, inexpensive, and well-tolerated substance, rich in calcium and protein. This regime allows the patients to become anabolic and to undergo laparoscopic reconversion under better conditions, a few months after placement of the gastrostomy.
Alternatively, after a complicated distal bypass, reversal to a more “normal” bypass with an AL of approximately 100 cm and a long CL is a possible remedial operation, but here as well we prefer to proceed to gastrostomy feedings for some time before the final reintervention.
Another option to correct excessive caloric intake after LRYGB is to enhance restriction. A common technique to achieve additional restriction is the placement of a band around the gastric pouch, preferably a few cm proximal to the GE, a technique that was popularized by Fobi [18]. The distance needed to accommodate the band may be a problem when dealing with a micropouch, especially when using an adjustable gastric band, as described by Bessler’s [19] team. We described the use of an adjustable band in conjunction with LRYGB some 8 years ago, but we abandoned this technique because of an unacceptable erosion rate. In fact, erosion has been a problem in our hands for the Fobi ring as well. This problem is not surprising because a foreign object placed across a staple line has the tendency to migrate inside the viscus. Erosion may be even more of a problem in redo cases because the additional dissection necessary to place the band in a correct position increases the chances of ischemia in addition to the already hazardous crossing of a staple line. We do not advocate the use of any type of band in LRYGB when the bypass was performed as a remedial operation. In case of erosion, the best approach is endoscopic removal [20]. Initially, we removed the eroded bands laparoscopically, but we experienced a high rate of leaks with this method.
Gagner’s team developed an interesting technique to achieve additional restriction without using a foreign object. The technique consisted of reducing the gastric pouch together with the proximal part of the AL by resecting both items longitudinally with a linear stapler, however with disappointing long-term results [21]. Rather than resecting the pouch and the AL longitudinally, we recently attempted to reduce both by plication around a 34 French orogastric tube held in close contact with the lesser curvature of the pouch and with the antimesenteric side of the AL, similarly to the technique of greater curvature plication [22], an experimental substitute for LSG. Our technique is promising in the short term, but it is too early to advocate it on a large scale.
Reversal of the Bypass
Reversal for Weight Issues
In some cases, the LRYGB does not live up to expectations. Some patients never lose significant weight with the operation. When weight loss is not satisfactory despite immediate postoperative maximal restriction, adding restriction by one of the techniques described previously is not a valid option. As for the malabsorption, the lack of weight loss indicates that the moderate malabsorption following LRYGB does not suffice. Thus, in patients who never lose weight after LRYGB, it may be appropriate to change the overall strategy and to reconvert the anatomy to the preoperative conditions and start from there [23]. Obviously, reconversion to normal anatomy will likely not improve weight loss. However, from the newly obtained normal anatomy, a laparoscopic sleeve gastrectomy (LSG) can be constructed, either after some delay or at the time of the conversion. We believe that for some patients, the restrictive aspects of LSG may be superior to LRYGB. The presence of a functional pyloric sphincter in addition to the highly reduced gastric volume influences gastric emptying and has proven to interfere with Ghrelin production, whereas the dumping syndrome is usually less of an issue. In addition, in cases in which restriction still does not have the expected results, the LSG can logically be complemented by a duodenal switch (DS). DS has been shown to be the most efficient weight loss operation and to provide the best surgical results in terms of type 2 diabetes mellitus (T2DM) control [24]. The combination of a relatively normal stomach (although reduced in volume) with a malabsorptive construction is likely to be safer than a distal gastric bypass. In addition, the surgeon may ascertain that the patient, submitted to DS as a third- or even fourth-stage surgery, is compliant enough with follow-up to undergo an operation that is highly demanding for the patient.
Reversal for Glucose Metabolism Issues
When we reviewed our long-term (9 years) results of LRYGB, we found some odd outcomes in terms of glucose metabolism. Similar to the weight results, long-term issues of glucose metabolism appeared to be linked to poor dietary habits the patients had developed, in conjunction with a suboptimal follow-up. We found that, whereas patients with existing T2DM did well after LRYGB and experienced a marked improvement in their condition, more than 25 % of the non-affected patients developed new-onset T2DM. In addition, over 30 % of the patients developed frequent hypoglycemic symptoms late after LRYGB, and some were hospitalized for seizures linked to recurrent low plasma glucose [25]. Hypoglycemic syndrome or neuroglycopenia (NGP) is due to the excessive secretion of insulin, secondary to the ingestion of simple sugars. When hypoglycemia cannot be corrected by dietary measures or by the medication that is usually prescribed for this condition, such as octreotide and acarbose, surgical revision may be contemplated. A safe and effective approach is reversal of the bypass to a normal anatomy. Before proceeding to reconversion, it is prudent to perform gastrostomy as a diagnostic test to confirm the efficacy of eliminating the deleterious effect of the duodenal exclusion inherent with the bypass. When, due to the gastrostomy, feedings along the normal digestive pathway appear no longer to be accompanied by postprandial hypoglycemia, laparoscopic reconversion may be performed. We practiced the principle of undoing the duodenal exclusion in a patient who developed new-onset insulin-dependent T2DM and who displayed extreme glycemia fluctuations in connection with poor dietary habits that proved to be unmanageable. Reversal to a normal anatomy in this patient resolved the fluctuations and unexpectedly, actually appeared to cure the T2DM in the short term, as demonstrated by a normal oral glucose tolerance test (OGTT) performed approximately 4 weeks after reconversion.
Preparing for the Reintervention and Choosing the Type of Remedial Operation
Except for the cases in which an anatomical flaw is evident and in which surgery constitutes a first-choice solution, it is essential to diagnose the problem leading to unsatisfactory results after LRYGB. The decision to reintervene, either laparoscopically or endoscopically, must be made by a multidisciplinary team. In our practice, this team consists of a dietitian, a psychologist, a gastroenterologist, a radiologist, an endocrinologist, and a bariatric surgeon.
The multidisciplinary consultation is organized by the bariatric coordinating nurse, who personally knows the patients and who can evaluate the capacity of the patient to deal with dietary restrictions, lifestyle changes, compliance with medication, or reoperation. She constitutes the first and most important selection agent. The psychologist’s input is invaluable as well, as this professional specifically analyzes how the patient has progressed since the preoperative interview. In some instances, the patient will demonstrate a psychological incapacity to deal with the features of the bypass. In some cases, the psychologist will need to demonstrate that the expectations of the patient were unrealistic and teach the patient to accept the results of the bypass, even when they do not match the anticipated outcome. In addition, the psychologist evaluates the patient’s psychological power to bear yet another operation, if needed.
The dietitian analyzes the subject’s dietary adjustment to the bypass construction. There are three specific ways to adapt to the reduced caloric intake after RYGB:
1.
If restriction is still active, a patient can increase the number of meals (grazing, polyphagia).
2.
Conversely, if restriction has been weaned, the patient may increase the size of meals (hyperphagia).
3.
Finally, the patient sometimes can no longer observe the dietary restrictions that are part of the postoperative recommendations.
Typically, patients no longer experience a dumping phenomenon some years after the bypass and become “sweets eaters.” This is an essential point, because the dumping syndrome, according to Sugerman [26], is one of the most important mechanisms for persistent weight loss after RYGB. When the syndrome is eliminated, patients have the tendency to eat more sweets, even more so when restriction is still active. We have found (submitted data) that sweets eating typically appears as a consequence of a poor follow-up. According to Scopinaro, a sweets-eating behavior cannot be addressed by surgical measures. Sugar eaters, therefore, rely almost entirely on appropriate dietary counseling. For polyphagia and hyperphagia, a surgical solution can be chosen that takes this dietary evolution into consideration. Polyphagists cannot be helped by additional restriction and will best be helped by a malabsorptive alteration of the bypass. Alteration may consist of distalization of the bypass (transection of the AL at the enteroenterostomy and reimplantation some 150 cm proximal to the cecum); alternatively, reconversion to normal anatomy may be performed, with immediate or delayed sleeve gastrectomy, followed by DS. The choice will often depend on the presence of GERD. In cases of reflux, we are reluctant to construct a sleeve gastrectomy, a construction that is known to induce GERD. An additional argument against choosing the sleeve solution is that simple reconversion to normal anatomy results in a significant prevalence of GERD [27]. Therefore, the most common solution in polyphagists who experience weight regain or obesity recidivism is distalization. Conversely, for hyperphagists, the RYGB can be complemented by an additional restrictive measure. As previously mentioned, this restriction may include the placement of a band, adjustable or not, in non-revisional LRYGB or the refashioning of the existing construction, with resection or plication of the pouch, anastomosis, and AL—an option that is preferred in redo-RYGB.
The gastroenterologist




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






