Reference
Type of procedure
Mean preop BMI (kg/m2)
%EWL
T2DM remission (%)
Follow-up (months)
O’Brien et al. [6]
LAGB
33.7 (32.9–34.4)
87.2
N/A
24
Dixon et al. [5]
LAGB
37 (22 % BMI < 35)
62.5
73
A1c < 6.2 %, no meds
24
Sultan [8]
LAGB
33 (28–40)
70
50
No meds
24
Lee et al. [9]
SG
31 (25–35)
69.1
50
A1c < 6.5 %, no meds
12
Lee et al. [10]
SG
28.5 (22–35)
BMI reduction to 24.8
55
A1c < 6 %, no meds
12
Abbatini et al. [11]
SG
32.7 (28–35)
BMI reduction to 21.1
88.8
A1c < 6.5 %, no meds
12
Schauer et al. [12]
SG
36.2 (36 % BMI < 35)
81
37
A1c < 6 %
12
Cohen et al. [13]
DJB
28.4 (23.9–34.9)
BMI reduction to 25.2
40
A1c < 7 %, no meds
12
Cohen (to be published)
Sleeved DJB
28.9 (25–35)
6.8 (total WL)
71
A1c < 7 %, no meds
12
Cohen et al. [16]
RYGB
32.5 (32–34.9)
81
97
A1c < 6 %, no meds
20
Lee et al. [17]
RYGB
30.1 (23–35)
28.8
80
A1c < 6.5 %
24
Lee et al. [10]
RYGB
28.5 (22–35)
BMI reduction to 23.2
79.3
A1c < 6 %, no meds
12
Shah et al. [18]
RYGB
28.9
(22–35)
BMI reduction to 23
100 %
No meds
9
Schauer et al. [12]
RYGB
37 (28 % BMI < 35)
88
42 %
A1c < 6 %
12
Scopinaro et al. [19]
BPD
30.6 (25.3–34.9)
BMI reduction to 25.6
83 %
A1c < 7 %, no meds
12
Chiellini et al. [20]
BPD
30.9 (26–33)
BMI reduction to 25.1
100 %
A1c < 7 %, no meds
18
De Paula et al. [21]
II + SG with or without duodenal exclusion
30.3 (25–35)
BMI reduction to 23.5
86.4 %
A1c < 7 %, no meds
39
Laparoscopic Adjustable Gastric Banding (LAGB)
The landmark trial for laparoscopic adjustable gastric banding (LAGB) versus intensive medical treatment, published by O’Brien et al. in 2006, included 80 patients with a BMI between 30 and 35 kg/m2 and found that the patients in the surgical group had 87.2 % excess weight loss (EWL), whereas the nonsurgical group had 21.8 % EWL. Improvement of metabolic syndrome was also observed in the surgical group [5]. Two years later, Dixon et al. published a randomized controlled trial that analyzed the results of LAGB in diabetic patients with a low BMI (>30 and < 40 kg/m2). This study compared the results of LAGB and conventional medical therapy in 60 patients with early-onset (less than 2 years) T2DM. Only 13 (22 %) patients had a BMI < 35 kg/m2 (6 underwent surgery and 7 underwent medical therapy). Diabetes remission (fasting plasma glucose [FPG] < 126 mg/dL and glycated hemoglobin [HbA1c] < 6.2 %, without antidiabetic medication) was observed in 73 % of the patients in the surgical group and 15 % in the control group, and a 20 % weight reduction (62.5 % EWL) was observed in the surgical group versus 1.4 % (4.3 % EWL) in the control group. A greater percentage of weight loss at 2 years and a lower baseline HbA1c were independently associated with remission. The authors also observed a significantly greater improvement in insulin resistance and in the levels of triglycerides and high-density lipoprotein (HDL) cholesterol in the surgical group. Despite this improvement in glycemic control in patients with BMI between 30 and 35 kg/m2, few participants achieved remission with a body weight loss of less than 10 %; therefore, the major driver of glycemic improvement following LAGB is weight loss. Moreover, the efficacy in achieving T2DM remission should be analyzed carefully because all patients in this study had early-onset T2DM and were followed for a short time (2 years) [6].
Sultan et al. published in 2009 the first US results for LAGB in patients with a BMI of 30–35 kg/m2 and observed 70 % EWL at 2 years. The authors also observed significant resolution and improvement of comorbidities, although the criteria used were based on the medications prescribed by the physician [8].
In summary, LAGB promotes greater weight loss than intensive medical care. It also results in the resolution or improvement of diabetes and other comorbidities; however, such changes are directly related to the weight loss, and long-term results are needed before we can validate this technique for low-BMI patients. To obtain optimal results regarding diabetes treatment, LAGB should only be considered in patients with early-onset diabetes (less than 2 years).
Sleeve Gastrectomy
The initial belief that sleeve gastrectomy (SG) is only a restrictive operation is controversial, to say the least. Many published studies verify the superiority of SG when compared with medical treatment and pure restrictive procedures, such as LAGB. SG may have some hormonal background that underlies the early postoperative improvement in insulin sensitivity, but there may be some confounding factors such as caloric restriction, which is a well-established mechanism for T2DM improvement.
When compared with Roux-en-Y gastric bypass (RYGB) or biliopancreatic diversion (BPD), SG promotes less weight loss, and the comorbidity remission rate is lower, which leads many surgeons to adopt this technique for non-morbidly obese patients to prevent the questionable risks of excessive weight loss. However, because SG in patients with a low BMI has only been performed recently, reliable follow-up data are scarce.
Lee et al. published in 2010 the first case series of SG in 20 diabetic patients with a mean BMI of 31 kg/m2 and observed 69.1 % EWL, with a 30 % reduction in HbA1c and T2DM resolution in 50 % of the patients (FPG < 126 mg/dL and HbA1c < 6.5 % without any glycemic therapy) at 1 year. Lee et al. concluded that SG may be less effective for T2DM resolution in patients with overweight or Class I obesity (BMI > 25 kg/m2 and < 35 kg/m2) than in patients with morbid obesity (BMI > 35 kg/m2). It has to be noted that in Asian populations, because of several factors such as alimentary habits and genetics, patients with a lower BMI have metabolic profiles/comorbidities similar to those that occur in Caucasian patients with a higher BMI. Poorly controlled diabetes, high preoperative HbA1c levels, and a higher incidence of β (beta) cell failure observed in mildly obese patients were some of the reasons mentioned by the authors for the lower rate of T2DM remission [9].
In another study that included 200 diabetic patients with a BMI below 35 kg/m2 who were submitted to different surgical procedures, the rate of DM remission (FPG < 110 mg/dL, HbA1c < 6 % without any medication) in patients submitted to SG (24 out of 200) was 55 % at 1 year, with little weight loss. The most important predictor of T2DM remission in these patients was the duration of the disease. Patients with a higher BMI (30–35 kg/m2) had better results than those with a BMI below 30 kg/m2, probably because of increased insulin resistance [10].
More recently, Abbatini et al. compared the results of SG and medical treatment in 18 non-morbid obese diabetic patients. In the surgical group, the mean BMI was 21.1 kg/m2 (27.5 % reduction) at 1 year, the T2DM resolution was 88.8 % (8 out of 9 patients), and a significant reduction of comorbidities was also observed. This effect could be weight loss independent, as the glucose changes occurred only a few days after surgery, before significant weight changes were observed.
The only patient who did not achieve remission had a long history of diabetes and was receiving high-dose insulin therapy. In the control group, there was no significant difference in the BMI or fasting glycemia before and 1 year after surgery. All patients continued their glycemic therapy [11].
It seems that SG is an interesting alternative for the treatment of diabetic patients with a low BMI; however, the duration of the T2DM may be a crucial factor when selecting this type of procedure, as poorer results have been observed in patients with a long history of T2DM. Recent studies have shown favorable results in comparisons with medical treatment and LAGB. When compared with RYGB, SG promotes less weight loss and glycemic control, but the differences seem to be modest.
Is SG a good option for diabetic patients with low BMI? Some surgeons would agree by stating that SG is a less complex surgery, with lower morbidity and mortality, and that is associated with a lower incidence of nutritional deficiencies in the long term. We cannot agree entirely with such assumptions before long-term data and new randomized control trials (RCTs) are available to the medical community.
Schauer et al. reported a series of 150 diabetic patients with BMI values from 27 to 43 kg/m2 who were randomly assigned to three groups (medical therapy, RYGB, and SG). The primary outcome was the percentage of patients who achieved HbA1c < 6 %, regardless of the use of T2DM medications, 12 months after randomization. The mean BMI was 36 kg/m2, and 18 (36 %) patients in the SG arm had a BMI of less than 35 kg/m2. The target HbA1c level of 6 % was achieved in 12 % of the medical therapy group versus 37 % of the SG group and 42 % of the RYGB group. There were no significant differences between the two surgical groups; however, 28 % of the patients in the SG group that achieved HbA1c < 6 % did so with the use of one or more glucose-lowering drugs, whereas none of the patients in the RYGB group required any type of additional medical therapy. The authors also demonstrated improved glycemic control and the reduction of other cardiovascular risk factors, such as dyslipidemia and hypertension. The lower rate of T2DM resolution when compared with other studies was attributed to the inclusion of patients with more advanced T2DM (mean duration of 8.5 years, mean HbA1c baseline level of 8.9–9.5 %, pre-interventional treatment with an average of three types of drugs, and a high number of patients under insulin treatment). Other reasons were less severe obesity, a higher proportion of male and black patients, and older age [12].
Operations Involving Duodenal Exclusion (Duodenal-Jejunal Bypass, Roux-en-Y Gastric Bypass, and Biliopancreatic Diversion)
T2DM remission after bariatric surgery varies according to the type of surgery, and better results are achieved by procedures that include anatomic diversion of the upper gastrointestinal tract when compared with restrictive procedures alone. Resolution of T2DM after RYGB and BPD typically occurs too quickly to be accounted for by weight loss alone, which suggests that these operations have a direct and more profound impact on glucose homeostasis.
Energy intake restriction and weight loss differences observed among different types of surgery may be accounted for the metabolic outcomes of bariatric surgery, but data in subjects with RYGB have shown greater improvement of glucose tolerance and insulin sensitivity when compared with subjects that lost the same amount of weight with diet therapy. As described previously, the improvement was observed weeks or even days after surgery and before any significant weight loss occurred.
The final common mechanism of T2DM resolution seems to be duodenal bypass, although some degree of more rapid food delivery to the distal intestine occurs in RYGB and BPD.
“Classic” Duodenal-Jejunal Bypass
Duodenal-jejunal bypass (DJB) prevents direct contact between ingested nutrients and the duodenum and proximal jejunum without any gastric restriction or exclusion and may be an alternative surgical therapy to promote glucose homeostasis without significant weight loss (Fig. 30.1).
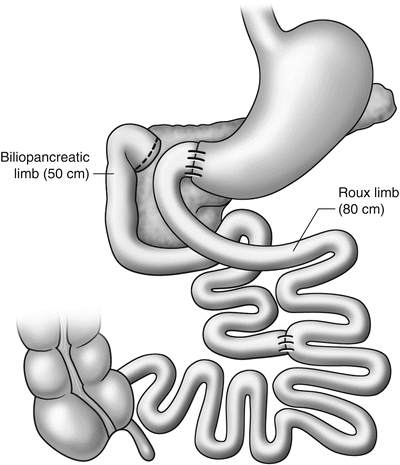

Fig. 30.1
Classic duodenal-jejunal bypass
In early 2007, we published a step toward extending animal studies into the clinical arena by reporting two cases of diabetic patients who underwent laparoscopic DJB. They were overweight or mildly obese, with BMIs of 29 and 30.3 kg/m2. T2DM was not particularly long-standing (2 and 7 years, respectively), and it was treated before surgery with insulin plus metformin in one case and with rosiglitazone and metformin in the other. Evaluations at 1 week, 1 month, and thereafter at monthly intervals for 9 months demonstrated rapid and unequivocal improvements in several simple measures of glucose control. FPG levels were initially in the diabetic range (148 and 178 mg/dL), but they decreased steadily after surgery, reaching nondiabetic values by 1 month and remaining at 100 mg/dL throughout postoperative months 3 through 9. Similarly, fasting insulin levels started high (27 and 29 mmol/L) but declined quickly and progressively after surgery, remaining at levels typical of individuals without diabetes (approximately 5 mmol/L) throughout postoperative months 3 through 9. Reflecting the improvement in glycemia, HbA1c values decreased from diabetic (8–9 %) to normal (5–6 %) values by 3 months, and they remained equally low thereafter during the remaining 6 months of observation. A key finding was that this salutary transformation occurred with no weight loss in either patient.
We performed a longitudinal prospective study of 35 overweight diabetic patients. Thirty-five subjects (20 men and 15 women) were included in this study. The mean preoperative BMI was 28.4 kg/m2. Laparoscopic DJB was performed in all patients, and anthropometric data and blood samples were collected at baseline (preoperative) and 3, 6, 9, and 12 months after surgery. Success was defined when patients reached HbA1c levels < 7 % without diabetic medications.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






