Fig. 20.1
Roux-en-Y gastric bypass
In general, obstruction of the Roux limb or common channel will result in obstructive symptoms familiar to the general surgeon, including nausea, vomiting, food intolerance, abdominal pain, and distention. Obstruction of the biliopancreatic limb is more challenging to diagnose, since the alimentary path may remain unblocked. In biliopancreatic limb obstruction, the gastric remnant may become severely distended with fluid yet remain invisible on plain film (Fig. 20.2a). This may result in abdominal fullness, bloating, hiccups, and pain but no nausea or vomiting. For this reason, CT scan of the abdomen and pelvis is a critically important component of the evaluation of the patient with possible bowel obstruction, as it will reveal obstruction of the both the bypassed and non-bypassed bowel (Fig. 20.2b). Obstruction of the biliopancreatic limb will result in severe distension of the gastric remnant and generally requires emergent decompression, either surgically or percutaneously.
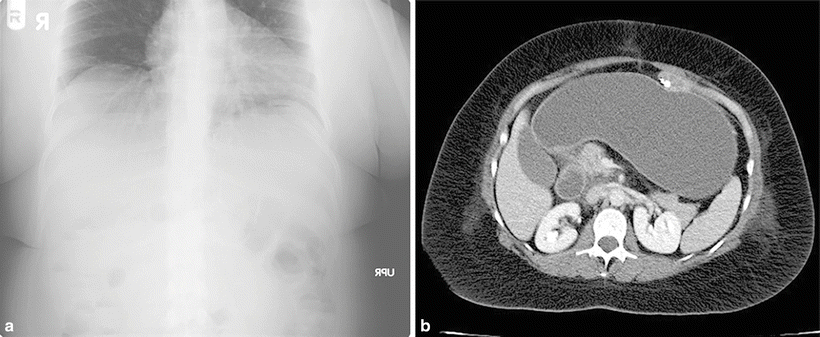

Fig. 20.2
(a) This plain film of a gastric bypass patient with obstruction of the biliopancreatic limb is not particularly revealing. (b) CT imaging of the same patient reveals a tremendously enlarged gastric remnant filled with fluid. The antegastric Roux limb containing a small amount of contrast can be appreciated anteriorly
Gastrojejunal Stricture
Stricture of the proximal anastomosis, or gastrojejunostomy, is one of the most common complications after gastric bypass. In a 2006 study reviewing 1,291 patients at the Cleveland Clinic Florida, 7.3 % developed strictures requiring intervention [1]. While a significant majority of gastrojejunal strictures present within the first 90 days after surgery, some patients may present much later, even a year or more postoperatively [2]. The typical stricture patient presents 4–6 weeks after surgery with solid food intolerance progressing to liquid intolerance as the stricture narrows. In severe cases, patients may be unable to swallow their own oral secretions.
The diagnosis of stricture can usually be made based on history alone and confirmed with upper endoscopy. Radiographic contrast studies are not usually helpful; they are not very sensitive in detecting strictures and pose the risk of contrast aspiration. Upper endoscopy is the primary diagnostic modality of choice, as it allows both rapid diagnosis and therapeutic intervention via balloon dilatation [3]. It is difficult to precisely measure a stricture, and no formal definition of stricture exists, but most endoscopists consider an anastomosis that is too narrow to permit passage of a standard upper endoscope (approximately 9.5 mm in diameter) to represent a stricture. After being diagnosed endoscopically, the stricture can be immediately dilated using a through-the-scope dilating balloon up to a diameter of 12–15 mm. While most patients respond to a single dilatation, some will require a second or third; up to 13 % may require four to five treatments [4].
Balloon dilatation is a safe procedure but perforation may rarely occur. In a 2008 study of 61 patients who underwent 128 dilatations, 3 patients experienced radiographic evidence of perforation afterwards [4]. These patients were immediately taken to the operating room for laparoscopic exploration; although the site of the perforation was not found in any of the 3, all of the patients in this series responded well to surgical drainage, bowel rest, and intravenous antibiotics.
The etiology of gastrojejunal stricture formation is not well understood. Some believe it is related to ischemia while others feel that suture material or staples may be causative. Stricture formation appears to be related to the initial size of the anastomosis; a 2003 study showed that switching from a 21 mm circular stapler to a 25 mm circular stapler reduced the rate of stricture by a factor of 3, from 27 to 9 % [5]. For stapled anastomoses, firm apposition or compression of the tissue edges may be helpful in reducing stricture rate. A recent study demonstrated that a circular stapler with 3.5 mm staple height resulted in a lower stricture rate than one with 4.5 mm staples [6]. The use of staple line reinforcement materials has also been shown to reduce stricture rate [7]. Some surgeons feel that handsewn anastomoses are less likely to stricture, while others prefer linear stapled or circular anastomosis; no single study has convincingly supported one of these approaches as superior.
Although obstruction at the distal anastomosis is sometimes described as a “distal stricture,” these obstructions are most likely due to kinking of the anastomosis or excessive narrowing of the common enterotomy closure due to technical error. True stricture formation along a 60 mm stapled jejunojejunostomy anastomosis is exceedingly rare.
Obstruction from Internal Hernia
Internal hernias are relatively common after gastric bypass and may result in bowel obstruction, intestinal ischemia, or both. A 2007 series from the Cleveland Clinic reported that internal hernia was the single most common cause of bowel obstruction in their gastric bypass patients, representing 41 % of all obstructions [8]. In a gastric bypass patient with an antecolic Roux limb, two different types of internal hernias may potentially occur:
Distal anastomosis mesenteric hernia—this space is bordered by the divided mesentery of the biliopancreatic limb and the mesentery of the Roux limb at the distal anastomosis (Fig. 20.3).
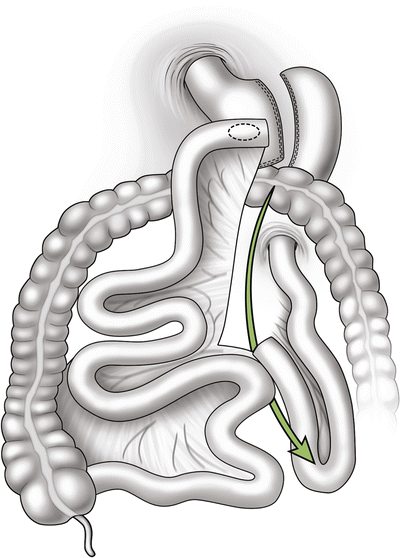
Fig. 20.3
Internal hernia potential space at the mesenteric defect of the distal anastomosis
Petersen hernia—the space between the mesentery of the Roux limb and the mesocolon/colon (Fig. 20.4).
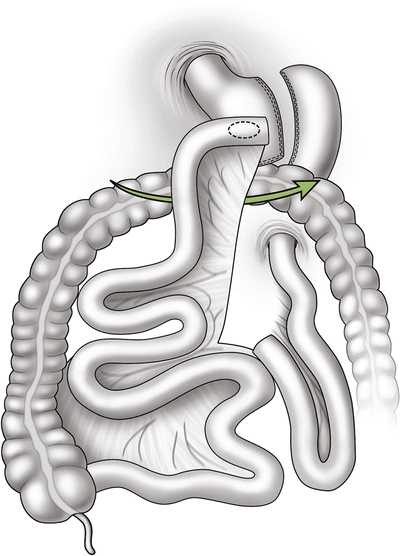
Fig. 20.4
Internal hernia potential space at between the Roux limb mesentery and the mesocolon, frequently referred to as a Petersen hernia
Some bariatric surgeons perform a retrocolic gastric bypass in which the Roux limb is brought up behind the transverse colon through an opening in the mesocolon. These patients have the potential to suffer from a “mesocolic hernia” in which the Roux limb herniates up through the defect in the mesocolon through which the Roux limb passes. If the Roux limb is placed in an antecolic position, no such opening is created, and there is no potential for mesocolic herniation.
While the majority of bariatric surgeons close these internal hernia spaces with permanent suture, some do not. A Mount Sinai study from 2005 found that sutured closure of the defects reduced the internal hernia rate from 3.3 to 1.2 % [9]. It is important to remember that even if hernia spaces have been sutured closed, hernia defects may still form at these sites.
If small bowel becomes entrapped within an internal hernia, its venous outflow may become partially or completely occluded, ultimately resulting in bowel ischemia and severe abdominal pain far out of proportion to the physical exam findings (Fig. 20.5). Typically, patients complain of intense pain in the midepigastrium, often radiating to the back. The pain may occasionally be relieved by leaning forward or getting “down on all fours,” maneuvers that serve to reduce the compression on the entrapped bowel. Entrapped bowel may reduce itself from the hernia spontaneously, bringing with it rapid resolution of symptoms, or it may persist until surgical intervention. Obstruction of the bowel lumen may or may not occur at the same time (Fig. 20.6).
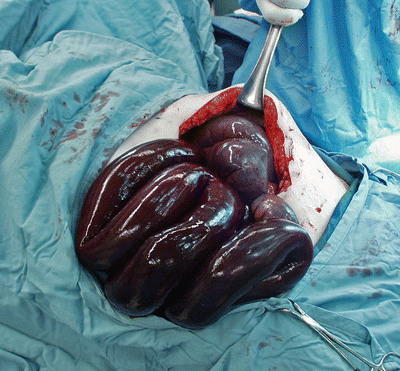
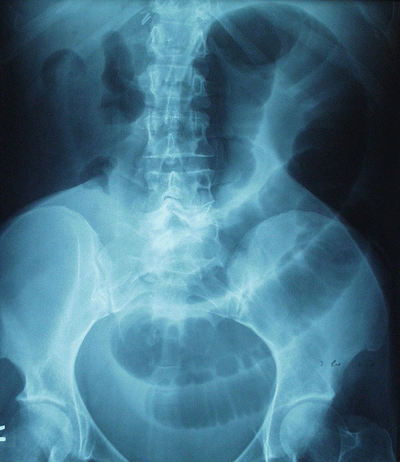

Fig. 20.5
Petersen-type internal hernia after biliopancreatic diversion with duodenal switch, resulting in irreversible small bowel ischemia

Fig. 20.6
Plain film of a patient with an incarcerated internal hernia, showing dilated small bowel loops. Bowel obstruction may or may not be present with internal hernia
Physical exam is generally unrevealing in the patient with an internal hernia. Laboratory studies are similarly unhelpful. Computed tomography (CT) scan may demonstrate findings such as the “swirl sign,” a spiraling of the mesentery, abnormally bunched-up bowel loops in hernia locations, or intestinal obstruction if present. However, these findings are neither sensitive nor specific, and it is essential to understand that a negative CT scan of the abdomen in no way rules out an internal hernia. Bowel obstruction may be absent even with a severe internal hernia causing intestinal ischemia. Thus, the gastric bypass patient with severe unrelenting abdominal pain but a normal CT scan still represents a surgical emergency.
Definitive diagnosis of internal hernia can only be achieved through surgical exploration, either laparoscopic or open. If a significant length of bowel is entrapped within an internal hernia, intestinal anatomy may become so distorted that it becomes difficult to reduce the herniated bowel. In this situation, it is helpful to start by locating the ileocecal junction, then running the bowel in a retrograde fashion until the distal anastomosis is identified and the anatomy clarified. Once the herniated bowel is reduced, the hernia defect should be securely closed with a nonabsorbable running continuous suture. Some surgeons advocate the addition of a second layer of suture material or fibrin sealant to reinforce the hernia closure. If irreversibly ischemic bowel is identified, it will need to be resected; questionably viable bowel may warrant a “second-look” procedure within 24–48 h.
Small Bowel Obstruction from Scars and Adhesive Bands
The surgeon should always remember that bariatric surgery patients are also general surgery patients and may suffer from intestinal obstruction due to intra-abdominal adhesions. In a review of bowel obstruction after gastric bypass, Hwang et al. found that the single most common cause of obstruction was adhesive bands, representing 25 % of the 55 obstructions that occurred in their series of 1,715 patients [10]. Koopman’s review of 16 series of bowel obstruction after laparoscopic Roux-en-Y gastric bypass suggested that adhesions were the second most common source of obstruction (22 %) after internal hernia (42 %) [11].
Adhesions may form anywhere within the abdomen and can potentially obstruct the Roux limb, biliopancreatic limb, or common channel. Thus, plain films and even upper GI series may miss adhesive bowel obstructions that occur outside the alimentary path. CT scan with oral contrast is preferred since it will image the entire intra-abdominal gastrointestinal tract and may reveal the location of a transition zone where the obstruction is located. In the Cleveland Clinic series, plain films identified 35 % of obstructions, while CT had a sensitivity of 51 % [8].
If the Roux limb is placed in a retrocolic position at the time of surgery, it must necessarily pass through a surgically created defect in the mesocolon. This opening may potentially scar to the point of stricture formation. In the Cleveland Clinic series, this was the second most common cause of bowel obstruction in bypass patients, comprising 20 % of all obstructions [8]. The risk of mesocolic stricture formation or mesocolic hernia can be completely eliminated at the time of initial surgery, if the surgeon places the Roux limb in an antecolic position.
Incisional Hernia
Incisional hernias may form at open incisions or laparoscopic trocar sites or may exist as a sequelae of earlier surgery. Hwang’s review of 1,715 laparoscopic gastric bypass patients included 2 patients with early obstruction (<3 weeks post-op) at a trocar site, 1 patient with obstruction at an umbilical hernia site, and 1 patient with obstruction at a previous open incision site [10]. To minimize this risk, it is generally recommended that surgeons close all trocar sites 10 mm and larger. While this will not eliminate the risk of trocar site herniation entirely, it may significantly decrease the incidence.
The treatment of preexisting ventral hernias at the time of laparoscopic Roux-en-Y gastric bypass is more controversial. Most surgeons are reluctant to implant prosthetic mesh to repair large hernias during a clean-contaminated operation. Others feel that it is most prudent to wait until after the patient has achieved significant weight loss before performing the repair. However, some data are beginning to be published supporting simultaneous repair. A recent publication from Coimbatore, India, reported a series of 37 patients who underwent concomitant permanent mesh repair of ventral hernia at the time of bypass and sleeve gastrectomy, with excellent results [12]. While promising, bariatric surgery with concomitant mesh repair of ventral hernia does not yet constitute the standard of care.
Intussusception
Small bowel intussusception is a far less common cause of bowel obstruction after gastric bypass, with scattered case reports in the literature. In theory, the distal anastomosis can serve as a lead point for intussusception. If CT imaging can be obtained during the obstruction episode, the classic finding of the “target sign” will be pathognomonic for the condition. Intussusception may or may not resolve spontaneously. If surgery is required, the intussusception should be reduced, with resection or revision of the presumed lead point if identifiable.
Obstruction from Intraluminal Blood Clot or Bezoar
Intraluminal causes of obstruction are exceedingly rare after gastric bypass. Koopman’s review in 2008 identified only ten reported cases [11]. Intraluminal blood clots, or hemobezoars, may form in the immediate postoperative period and can cause occlusion at the distal anastomosis. If the location of the bezoar is accessible endoscopically, the obstruction can be relieved nonsurgically. Otherwise, surgical exploration may be required with evacuation of the bezoar and confirmation of hemostasis.
General Approach to the Bypass Patient with Obstruction
When approaching the gastric bypass patient with intestinal obstruction, it is important to remember that the clinical presentation is completely dependent upon the segment of bowel that is obstructed; an obstruction of the Roux limb will present far differently from an obstruction of the biliopancreatic limb. CT imaging with oral and IV contrast should be considered early in the diagnostic process as it will reveal obstruction in the Roux limb, biliopancreatic limb, and common channel. In contrast, plain films and upper gastrointestinal (UGI) series will generally be unhelpful in diagnosing obstruction of the bypassed bowel.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






