Revision Augmentation with Anatomic Form-Stable Silicone Gel Implants
Mitchell H. Brown
Introduction
Revision breast augmentation can be a very difficult and challenging undertaking. The normal anatomy that exists in a primary procedure is no longer present. The surgeon must deal with a variety of factors, some predictable and some not predictable. These include external and internal scarring, previously dissected tissue planes, alteration to the deep and superficial blood supply to the breast, alteration to the pectoral muscle, thinning and stretching of the overlying soft tissues, and secondary effects related to the presence of a capsule around the preexisting implant.
There are many possible reasons that a woman presents for secondary implant surgery. The most common seems to be for the management of a capsular contracture (1). Implant malposition is another common presentation and can be divided into malpositions that are superior, inferior, lateral, or medial (Fig. 131.1). Other common indications include management of rippling or implant edge palpability, size change, and the correction of soft tissue changes secondary to hematoma, infection, radiation, or previous surgery. Regardless of the presentation, the management of the secondary implant patient requires a systematic approach to identify the underlying problems, recognize the limitations, and develop a plan that is safe and maximizes the chances for a predictable outcome.
Implant selection in revision augmentation is a key component in achieving a good result. Many options exist, including round saline-filled implants, smooth and textured-surface, round gel implants, and textured, shaped gel implants. The shaped implant is a unique device that offers many potential benefits in the revision patient. Shaped gel implants have been available in one form or another since the 1960s. It was not until the early 1990s, however, that these implants gained widespread acceptance. Their use in primary breast augmentation (2) and in postmastectomy breast reconstruction is well documented (3). Shaped implants are indicated in patients who will benefit from the use of an anatomic device and who require an implant with form stability and are having the implant placed in a pocket that can be precisely controlled with respect to its dimensions.
The benefits of shaped, form-stable cohesive gel implants include their ability to provide a natural and proportionate breast shape (Fig. 131.2); the availability of a wide variety of shapes and sizes to match most breast shapes; maintenance of shape and upper pole fill, which translates into a decreased likelihood of rippling; and a more viscous gel, which is less likely to escape from the implant shell, should the implant lose its integrity (Fig. 131.3) (3). Several studies have demonstrated a low rate of capsular contracture with form-stable anatomic gel implants (3,4,5). Allergan’s core data demonstrate a contracture rate in breast augmentation of 3.3% (5 year) with the Style 410 anatomic gel versus 15.5% (7 year) with round gel implants. In revision patients, the rates are 6.4% (5 year) for the anatomic implant and 20.4% (7 year) using a round device (6). Not only are the contracture rates low, but in addition the form-stable nature of the devices results in less deformation and clinical effect with a contracture compared to less-form-stable devices.
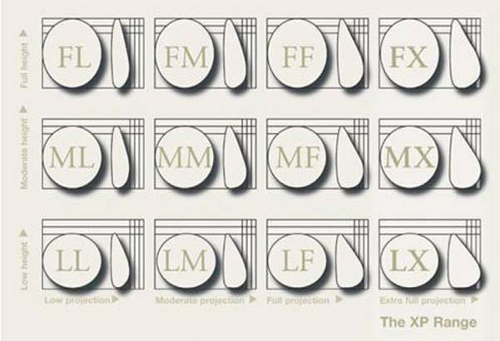
Shaped gel implants are available in a wide selection of shapes and sizes. These vary based upon implant width, height, and projection. The Natrelle 410 by Allergan (Irvine, CA) is one such shaped device. A 3 × 4 matrix based on height and projection provides the surgeon with great flexibility to custom select the implant based on precise patient measurements (Fig. 131.4). The texturing on this implant is designed to promote tissue adherence and implant stability within the breast pocket, a feature that is very helpful in secondary implant patients (Fig. 131.5). With smooth-surface implants, the device behaves as a free-moving object under the breast, which feels distinct and separate from the overlying breast tissue. This may result in unusual displacement of the implant with change in body position. With textured, form-stable gel implants, the resulting more adherent capsule results in a capsule, implant, and breast interface that behaves like a single unified breast mound. This “one breast feel” more closely resembles a normal breast and results in fewer secondary malposition issues (3).
Along with the Natrelle 410, the other anatomic gel implant that is commonly used in North America is the CPG by Mentor Corporation (Santa Barbara, California). This implant comes in three heights and three projections. The textured surface is produced with a negative imprint technique. This surface is less likely to result in tissue integration, therefore stability within the pocket is achieved through precise planning that matches the pocket dissection to the implant dimensions.
Indications
The decision to proceed with a revision breast augmentation should only be made after careful consideration of the patients concerns and a thorough discussion regarding goals and expectations. It is possible for the primary surgeon to decrease revision rates by committing to basic core principles that include patient education, informed consent, preoperative planning, proper implant selection using tissue-based planning, precise surgical technique, and structured postoperative care. Each indication for a revision can find its roots embedded in one or more of these principles. For example, a patient whose implant was improperly matched to a soft tissue envelope will likely develop implant malposition, rotation, or rippling. A patient who is concerned about an ongoing asymmetry but did not understand that this asymmetry existed preoperatively will not be prepared to accept the continued difference
in the two breasts. Surgery that uses imprecise technique increases the likelihood of infection, contracture, hematoma, and implant malposition. A device placed through too small an incision will be more likely to fail.
in the two breasts. Surgery that uses imprecise technique increases the likelihood of infection, contracture, hematoma, and implant malposition. A device placed through too small an incision will be more likely to fail.
The best way to manage a revision is to have avoided it in the first place. Having said that, it has to be acknowledged that errors do occur, soft tissues do not always behave as planned, well-intentioned decisions do not always turn out to be correct, patient’s bodies continue to change. and the principles of gravity continue to exist. For these reasons, the need for revision augmentation surgery will persist.
The first step in evaluating the revision patient is to develop a clear understanding of her concerns and expectations. This may be easier to explain for some patients than for others. Often, patients are abstract in the discussion of their concerns. Phrases such as, “this isn’t what I wanted,” “I am very unhappy with my breasts,” and “my friends tell me this can’t be right, it should be fixed” demonstrate displeasure with the outcome but are hardly specific. To correct a problem, one must understand what it is and why it occurred and have a reasonable plan for how to fix it. There is not a solution for every concern, nor is it reasonable to try to correct every outcome that is felt to be less than perfect.
The surgeon and patient should spend time performing a risk-benefit analysis. On one side of the equation are the reasons that the patient wishes a revision. The patient may have symptoms such as pain from a capsular contracture or a tight, poorly healed scar. She may have restriction of movement that affects her ability to do common activities. Other concerns may relate to the effect on quality of life. Asymmetry, improper sizing, malposition, or contracture may limit the wearing of certain clothing styles, and this may affect the patient’s self-image, self-esteem, and confidence, all reasons for having the augmentation done in the first place. These concerns must be balanced against the inherent risks and complications of the revision, as well as the likelihood for being able to meet the patient’s stated goals. The commonly used phrase, “the enemy of good is better,” should be carefully considered and discussed with the patient. There is nothing worse than performing surgery to correct a relatively minor problem only to be left with a major one.
Not all revisions of previous breast augmentations require the use of an implant. Patients who have had multiple previous procedures, painful contractures, implant ruptures, or complex malposition problems may be best served by implant removal with or without capsulectomy. Often, breast reshaping in the form of a mastopexy can be performed simultaneously or at a later date (Fig. 131.6).
When an implant is used for the revision surgery, careful consideration is necessary as to the type of implant to choose. This decision will be dictated by several factors, such as tissue
quantity and quality, desired aesthetic breast shape, need for form stability, ability to control the implant pocket, and patient preference. Shaped gel implants are an excellent choice in many revision cases. The form-stable nature of the gel allows the implant to assist in shaping the breast, a feature that is helpful in many secondary cases. When malposition of the implant is the presenting concern, the use of an implant with texturing will promote stability of the implant within the pocket. The low reported incidence of capsular contracture with the Natrelle 410 implant makes it an excellent choice in patients with problem capsules (3,4,5).
quantity and quality, desired aesthetic breast shape, need for form stability, ability to control the implant pocket, and patient preference. Shaped gel implants are an excellent choice in many revision cases. The form-stable nature of the gel allows the implant to assist in shaping the breast, a feature that is helpful in many secondary cases. When malposition of the implant is the presenting concern, the use of an implant with texturing will promote stability of the implant within the pocket. The low reported incidence of capsular contracture with the Natrelle 410 implant makes it an excellent choice in patients with problem capsules (3,4,5).
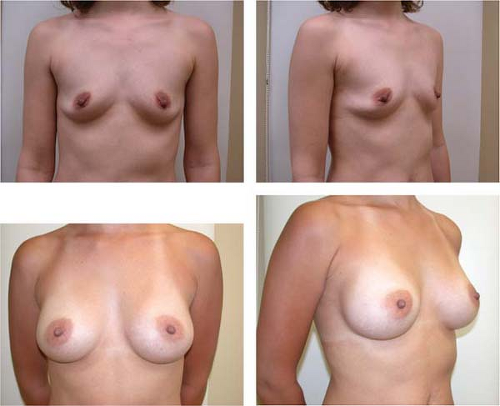 Figure 131.2. Before and 8-month postoperative views of breast augmentation with anatomic gel-filled implants, medium height, full projection, 295 g. |
Contraindications
As with any procedure, patients undergoing revision breast augmentation must have realistic expectations. There is not a solution for every problem, and in some circumstances it is best not to offer a surgical option.
Specific contraindications exist when using shaped gel implants in revision surgery. Of course, a patient must be willing to accept the use of a gel device. Outside of North America this is rarely an issue, but there a number of women in Canada and the United States who continue to have a greater comfort level with saline implants. Understanding a woman’s goals is key to selecting the best implant. Patients who would like a full-looking upper pole are not good candidates for a shaped implant. The form-stable nature of the gel results in a breast that is slightly firmer than what is seen with more responsive gel devices. Patients who are unwilling to accept this will do better with a softer round gel or saline implant.
The location and length of the surgical scar are important considerations for most implant patients. In revision surgery, there will be a preexisting scar. Surgery should always be performed through an incision that allows for direct access and precise correction of the underlying problem. If that cannot be done through the previous scar, then a new incision should be selected. When using a shaped gel implant, it is critical to have a long enough incision to allow for implant insertion without damaging the device. This is generally in the range of 5 cm. This incision length limits the choice for scar location. Most commonly, the incision will be placed in the inframammary fold or through a large periareolar incision. If an axillary approach is selected, it is imperative that the incision be of adequate length. Patients who are not willing to accept the required scar length and location are not candidates for shaped implants.
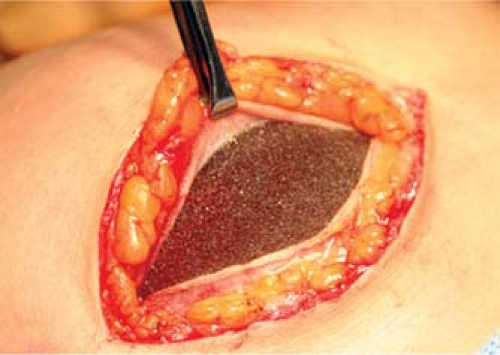 Figure 131.5. Adherence of a capsule to the surface of a Natrelle style 410 implant with Biocell surface. (Photograph courtesy of Dr. Bradley Bengtson.) |
One of the greatest advantages of shaped implants is their ability to provide differential fill with a tapered upper pole. Because of their shape, rotation is an issue that is unique to these implants. They must be placed in a pocket that is dissected specifically to match the implant dimensions. When shaped implants are used in revision surgery they must be placed in a new pocket. This can be achieved through a site change or by performing a capsulectomy. If implants are to be placed in a preexisting pocket, then shaped devices are contraindicated.
One of the fundamental principles of using shaped implants is to select a size based upon dimensional planning that pays attention to breast width, breast height, and the soft tissue envelope. This allows for filling out of the soft tissues with minimal risk of rotation. Patients who are not willing to select implant size based on these principles are not well suited for a shaped device.
Preoperative Planning
Careful preoperative planning is a cornerstone of any successful breast augmentation. This statement is especially true in revision surgery. It is absolutely imperative to have a detailed understanding of all previous breast surgeries. Whenever possible, surgical records should be obtained and reviewed. Often,
women presenting with breast implant problems have had multiple procedures. These may have included mastopexies, reductions, biopsies, or lumpectomies. Each of these surgeries will have left scars and have caused an alteration to the normal blood flow within the breast and to the nipple-areola complex. For each previous breast implant procedure, it is important to know the implant fill, surface, size, pocket, and incision used. Any complications such as infection, contracture, or malposition should be recorded, including the methods by which these complications were treated.
women presenting with breast implant problems have had multiple procedures. These may have included mastopexies, reductions, biopsies, or lumpectomies. Each of these surgeries will have left scars and have caused an alteration to the normal blood flow within the breast and to the nipple-areola complex. For each previous breast implant procedure, it is important to know the implant fill, surface, size, pocket, and incision used. Any complications such as infection, contracture, or malposition should be recorded, including the methods by which these complications were treated.
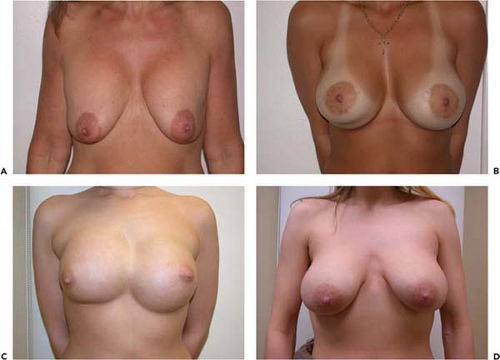
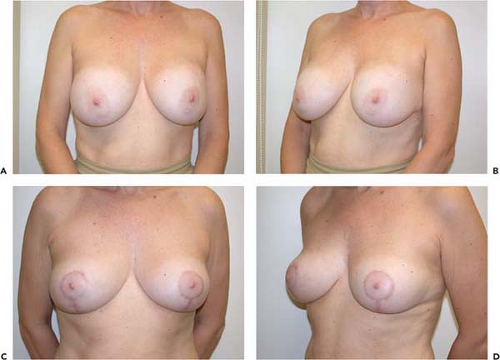 Figure 131.6. A, B: A patient with bilateral painful capsule contracture and adequate natural breast tissue. C, D: Six weeks following explantation, capsulectomy, and Wise-pattern mastopexy.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|