Chapter 32 Renal failure in association with thermal injuries
![]() Access the complete reference list online at http://www.expertconsult.com
Access the complete reference list online at http://www.expertconsult.com
![]() IN THIS CHAPTER
IN THIS CHAPTER ![]() PowerPoint Presentation Online
PowerPoint Presentation Online
Introduction
Acute renal dysfunction is a major complication affecting the thermally injured individual and is commonly associated with a high mortality rate. Currently, the incidence of acute renal failure in burn patients varies between 0.5% and 30%, with a reported mortality rate between 54% and 100%.1–10 Prior to 1965 there were no reported survivors from acute renal failure following burns.11 While advances have been made in the understanding of the etiology of acute renal failure in association with thermal injury, little has been accomplished with the actual treatment; and it remains unclear whether or not the application of renal replacement therapy (RRT) as a treatment modality has significantly changed the mortality rate of individuals suffering from acute renal failure.12 The best treatment for acute renal dysfunction is prevention, by understanding the pathophysiology of renal dysfunction in the thermally injured individual. This chapter will review the definition, etiology, pathophysiology, diagnosis, and treatment of acute renal dysfunction in association with thermal injury (Fig. 32.1).
Definition
Historically, acute renal failure has been defined as an abrupt and sustained decrease in renal function. Until recently, there was no consensus regarding an absolute definition for acute renal failure. This is exemplified by more than 30 different definitions having been used in the literature, creating much confusion and making comparisons between studies impossible.7,13,14 Although the definitions are numerous, the common theme of all definitions in the literature is an abrupt decline in glomerular filtration rate (GFR) with the inability of the kidneys to appropriately regulate fluid, electrolytes, and acid–base homeostasis. Furthermore, acute renal failure represents an end-stage process, with the phases leading up to it having important clinical and prognostic value. The need to grade the severity of injury, rather than consider only the most severe form, has led to a new classification system. The term acute kidney injury, or acute kidney insufficiency (AKI), is now preferred.14–16
In an effort to standardize the definition of renal insufficiency, the International Acute Dialysis Quality Initiative (ADQI) group has developed the RIFLE criteria (Fig. 32.2). The RIFLE criteria are a classification system that uses the GFR or urine output to define increasing levels of renal insufficiency. RIFLE defines three grades of acute kidney injury of increasing severity (risk, injury, failure), based on changes in either serum creatinine or urine output, as well as two outcome categories (loss and end-stage kidney disease). The RIFLE classification system has been validated in a number of clinical scenarios, including burn injury, with increasing grades corresponding to increasing mortality.5–10,17–19 In an attempt to include a time component for changes in creatinine, the ADQI have since modified the RIFLE criteria to the Acute Kidney Injury Network (AKIN) criteria (Table 32.1). The AKIN criteria define acute kidney injury as an abrupt reduction in kidney function (≤48 hours) and may allow for earlier recognition of AKI in the ICU setting.16 Comparisons between RIFLE and AKIN have been published but have not demonstrated clear benefit of one classification system over the other.20–22 These new classification systems should aid in future experimental design and allow for improved comparison between studies investigating AKI associated with thermal injuries.
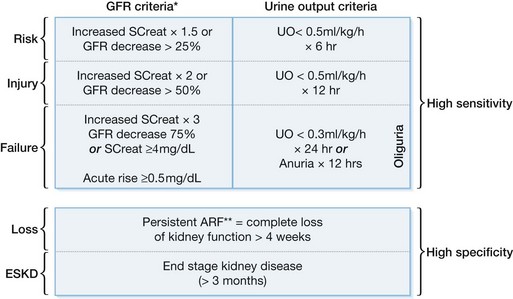
Figure 32.2 RIFLE classification scheme for acute renal failure (ARF).
(From Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, and the ADQI workgroup. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8:R204-R212.)
Table 32.1 RIFLE and AKIN criteria
| RIFLE category | Serum creatinine criteria | UO criteria |
|---|---|---|
| (A) The Acute Dialysis Quality Initiative (ADQI) criteria for the definition and classification of AKI (i.e. RIFLE criteria) | ||
| Risk | Increase in serum creatinine ≥1.5X baseline or decrease in GFR ≥25% | <0.5 mL/kg/h for ≥6 h |
| Injury | Increase in serum creatinine ≥2.0X baseline or decrease in GFR ≥50% | <0.5 mL/kg/h for >12 h |
| Failure | Increase in serum creatinine ≥3.0X baseline or decrease in GFR ≥75% or an absolute serum creatinine ≥354 µmol/L with an acute rise of at least 44 µmol/L | <0.3 mL/kg/h ≥24 h or anuria ≥12 h |
| AKIN criteria | Serum creatinine criteria | UO criteria |
| (B) The proposed Acute Kidney Injury Network (AKIN) criteria for the definition and classification of AKI | ||
| Stage 1 | Increase in serum creatinine ≥26.2 µmol/L or increase to ≥150–199% (1.5- to 1.9-fold) from baseline | <0.5 mL/kg/h for ≥6 h |
| Stage 2 | Increase in serum creatinine to 200–299% (>2–2.9 fold) from baseline | <0.5 mL/kg/h for ≥12 h |
| Stage 3 | Increase in serum creatinine to ≥300% (≥3-fold) from baseline or serum creatinine ≥354 µmol/L with an acute rise of at least 44 µmol/L or initiation of RRT | <0.3 mL/kg/h ≥24 h or anuria ≥12 h |
(From Brochard L, Abroug F, Brenner M, Broccard AF, Danner RL, Ferrer M, Laghi F, Magder S, Papazian L, Pelosi P, Polderman KH, on behalf of the ATS/ERS/ESICM/SCCM/SRLF Ad Hoc Committee on Acute Renal Failure. An official ATS/ERS/ESICM/SCCM/SRLF statement: prevention and management of acute renal failure in the ICU patient: an international consensus conference in intensive care medicine. Am J Respir Crit Care Med. 2010; 181(10):1128–1155.).
Etiology of acute renal failure
Burn-related renal insufficiency is most commonly observed during the period of initial resuscitation after burn injury (early AKI) or as a component of the multiorgan dysfunction syndrome, often associated with severe sepsis (late-onset AKI). The development of early AKI in the burn individual is multifactorial: hypovolemia, inflammatory mediators, cytokines, extensive tissue destruction and release of denatured proteins, iatrogenic causes (nephrotoxic agents), and cardiac dysfunction have all been implicated.3,4,23,24 Historically under-resuscitation and hypovolemia were the primary focus; however, more recent studies have demonstrated that AKI is not solely dependent on the amount of fluid received. AKI develops in the thermally injured patient despite aggressive fluid resuscitation and a normal urine output, and is more likely dependent on the degree of shock following injury. Global parameters of perfusion, i.e. lactate and base deficit, may better predict AKI, as they have been demonstrated to predict an increased risk of systemic inflammatory response syndrome (SIRS), acute respiratory distress syndrome (ARDS), multiorgan dysfunction syndrome (MODS), and mortality.5,25–29
Hypovolemia
Burns affecting >20% total body surface area (TBSA) are of sufficient size to induce decreased renal blood flow from local and systemic cytokine release as well as extravascular fluid loss.30,31 This concept is supported by the observations of Kim et al. that burn size is an independent predictor of acute renal failure in the burn population.32 Depressed renal blood flow results in ischemia and cellular death. The ischemic insult is known to produce oxygen free radicals that cause direct tubular damage, as well as disruption of tight junctions, resulting in obstructing casts which further reduce effective GFR. The duration of ischemic time is critically important to the development of AKI. Nguyen et al. found initial management of the thermally injured individual to be critically important to overall survival. Aggressive hydration had a protective effect against acute renal failure.33 Similarly the Shriners Burn Institute for Children, Galveston, observed that the time to initiation of resuscitative fluids was directly related to the incidence of renal dysfunction and overall mortality. They concluded that early aggressive fluid resuscitation lessens kidney damage and thus prevents renal dysfunction, which improves overall outcome.34
Hypervolemia, intra-abdominal hypertension, and abdominal compartment syndrome
Although aggressive fluid resuscitation may reduce the risk for AKI, it does not eliminate its occurrence. Studies have shown that AKI develops in burn-injured patients despite fluid resuscitation volumes in excess of that recommended by the Parkland formula, and despite normal average urine output (0.5–1.0 mL/kg/h).5 Furthermore, the risks of over-resuscitation have been well documented and include pneumonia, ARDS, compartment syndromes, and an overall increase in mortality.35–37
Burns >20% TBSA generally require intravenous resuscitative efforts, and the initial volume of fluids should be proportional to the area of burn injury. Despite a physician’s greatest effort to monitor endpoints of resuscitation, obligatory intercompartmental fluid shifts will occur during resuscitation.38 These intercompartmental fluid shifts can be particularly hazardous if they occur into fascial bound compartments, such as the peritoneal cavity. Numerous studies from the trauma literature have described the adverse physiologic effects of increasing intra-abdominal pressure on visceral perfusion.39–41 Intra-abdominal hypertension (IAH) is a known pathological process that may occur during initial burn resuscitation as defined by intra-abdominal pressures (IAP) >12 mmHg. Abdominal compartment syndrome (ACS) is defined as an IAP >20 mmHg with at least one concomitant organ failure. The exact incidence of ACS, the point of decreased visceral perfusion, is currently unknown during burn shock resuscitation.42–45 O’Mara et al. demonstrated that the volume and type of fluid resuscitation affects the development of ACS in the burn patient and suggested that fluid resuscitation with crystalloid >0.475 L/kg should alert the clinician to possible IAH/ACS and to monitor for decreased cardiac output, decreased lung compliance, or decreased renal perfusion – signs of ACS. In a mixed population of critically ill patients, a multicenter prospective trial has demonstrated that the occurrence of IAH during the ICU stay was an independent outcome predictor.45
Cardiac dysfunction
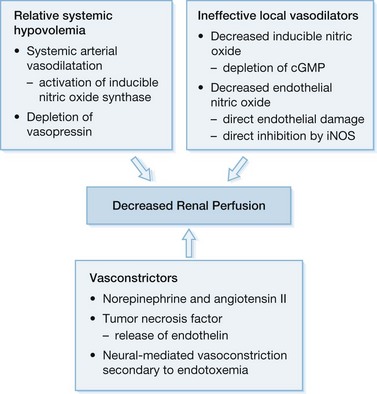
Cardiac dysfunction is known to result in reduced renal blood flow and hence to contribute to AKI. Although diminished cardiac output following thermal injury has been attributed to decreased preload or hypovolemia, there is increasing evidence of direct myocardial suppression. Myocardial dysfunction after thermal injury is commonly overlooked by physicians owing to the concentrated effort to correct the overwhelming state of hypovolemic shock and electrolyte abnormalities.46 An effective burn surgeon must rapidly re-establish adequate renal blood flow by correcting the diminished preload state while keeping in mind the impact of burn injury on the entire cardiovascular system. Patients suffering burns >50% TBSA are subject to decreased cardiac output, increased myocardial workload, myocardial ischemia, and acute cardiac infection from the large area of wounded skin.46–51 Several authors have suggested theories to explain the decreased cardiac output associated with thermal injury: 1) increased sympathetic activity with impaired adrenal response, 2) hypovolemia resulting in myocardial ischemia and 3) direct myocardial suppression.48–50,52–54 Of the potential theories, direct myocardial suppression by tumor necrosis factor (TNF, i.e. myocardial depressant factor) has gained substantial interest. TNF is known to be released by myocytes stimulated by endotoxin or direct thermal injury.55–61 The effects of TNF on cardiac function include reversible biventricular dilatation, decreased ejection fraction, and decreased stimulation to catecholamines (Fig. 32.1).57,62–63 Although most early cardiac dysfunction caused by TNF can be reversed by inotropic support, the key is early diagnosis to prevent ineffective renal perfusion and thus prevent the morbidity and mortality associated with renal insufficiency.
Denatured proteins
Rhabdomyolysis and free hemoglobin have both been implicated in the development of AKI and subsequent renal failure.64 Rhabdomyolysis in particular has been shown to contribute to AKI following severe burns. Rhabdomyolysis can arise secondary to direct thermal damage or compartmental syndrome and is commonly seen following severe electrical injury. The release of myoglobin, or unconjugated hemoglobin, into the systemic circulation results in blockage of renal tubules, constriction of afferent arterioles, and the generation of oxygen free radicals.65 The extent of renal injury is directly related to the amount of iron-containing molecules released, the state of hydration, and the degree of acidosis.66 Myoglobinuria occurs when serum myoglobin exceeds 1500–3000 ng/mL Elevated creatinine at baseline and creatine kinase levels >5000 U/L have been associated with the development of AKI and the need for RRT.67 Fortunately, the incidence of denatured proteins causing burn-associated acute renal insufficiency is low, and the overall prognosis is favorable if the pathological source is identified early and appropriate treatment is initiated.68 For those patients at risk, intensive hydration with isotonic crystalloids is recommended. The use of sodium bicarbonate is unnecessary, as it has not been shown to be superior to saline for alkalization of the urine.16,67
Sepsis
There have been significant advances in the treatment of burn injuries since 1965. Early aggressive resuscitation and excision have significantly influenced the course of acute renal failure immediately associated with thermal injury.69,70 Acute renal dysfunction associated with the septic syndrome nonetheless continues to cause significant mortality.71
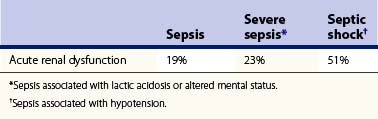
Sepsis and septic shock are the most common cause of death in the intensive care unit (ICU) and are seen in up to 87% of cases of acute renal dysfunction in the burn ICU.8,9 Several authors have found the degree of sepsis to be directly related to the incidence of acute renal dysfunction72,73 (Table 32.2). The key to treatment is an understanding of the pathophysiology of AKI associated with sepsis, the basis of which is multifactorial in nature but begins clinically with a generalized arterial vasodilatation secondary to a decreased systemic vascular resistance (Fig. 32.3). Initially, bacteria or their products activate sepsis-associated mediators (cytokines) locally at the site of direct invasion. In sepsis, the homeostatic balance between production and inactivation of these mediators is altered, allowing for systemic release, causing direct damage to the endothelium, vasoparalysis, and a procoagulant state. It has been theorized that acute renal insufficiency associated with sepsis is the result of each of these pathological processes. The vasoparalysis seen in sepsis results in a profound state of hypotension which activates the neurohumoral axis. In an effort to maintain systemic arterial circulation, the sympathetic nervous system and the renin–angiotensin–aldosterone axis respond by increasing cardiac output and by direct renal arteriolar vasoconstriction. This response may actually worsen renal perfusion by inducing a prerenal state, which is further aided by the release of other vasoconstricting agents (i.e. TNF, endothelin) as well as the inability of locally secreted vasodilators (endothelial and inducible nitric oxide) to counterbalance these sepsis-associated vasoconstrictors.71,74 Finally, as mentioned previously, sepsis induces a procoagulant state by affecting the expression of complement and the fibrinolytic cascade.75–77 This alteration in the homeostasis of coagulation may result in a state of disseminated intravascular coagulation (DIC) with direct injury to the kidney by glomeruli microthrombi.78 The net result is a lack of perfusion to the kidneys during sepsis that will ultimately culminate in acute tubular necrosis secondary to ischemic injury.
Diagnosis of acute kidney injury
Of the physiologic parameters of renal function, altered urine output is probably the first and most recognized sign of renal dysfunction. Urine output has been demonstrated to be a very specific but unfortunately not very sensitive measure of renal function.79 Most clinicians regard urine output of little diagnostic value in the evaluation of renal dysfunction, since severe renal injury may exist with any volume of urinary output. This variability is due to the fact that urine output is not determined by the GFR alone but by the difference between GFR and tubular reabsorption. The one clinical scenario in which urine output may be diagnostic is the presence of anuria (<50 mL/day) or complete cessation of GFR.80 The most common cause of anuria, excluding postrenal obstruction, is a severe prerenal condition. Although it is true that other conditions (acute cortical necrosis, bilateral arterial occlusion and rapidly progressive acute glomerulonephritis) may cause anuria, their incidence is low and the diagnosis is usually readily apparent owing to additional clinical signs.
Although urinary output is commonly non-diagnostic regarding the type of renal injury, microscopic and biochemical analysis may aid in the diagnosis and thus guide treatment options.81
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree