Symptomatic neuromas are a common cause of postamputation pain that can lead to significant disability. Regenerative peripheral nerve interface surgery is performed to treat symptomatic neuromas and prevent the development of neuromas. This review delineates the clinical problem of postamputation pain, describes the limitations of the available treatment methods, and highlights the need for an effective treatment strategy that leverages the biologic processes of nerve regeneration and muscle reinnervation. The evidence supporting use of regenerative peripheral nerve interface surgery to mitigate neuroma formation is discussed and the rationale behind the efficacy of regenerative peripheral nerve interfaces is explored.
Key points
- •
Painful neuromas are a substantial cause of morbidity in people with limb loss.
- •
Regenerative peripheral nerve interfaces may benefit patients with symptomatic postamputation neuromas through the use of free muscle grafts as physiologic targets for peripheral nerve reinnervation.
- •
Regenerative peripheral nerve interfaces should also be considered prior to amputation in order to prevent the development of symptomatic neuromas.
- •
Regenerative peripheral nerve interface surgery is a straightforward, reproducible procedure that can be effective in the prevention and management of symptomatic neuromas.
Introduction
In the United States alone, an estimated 2 million people live with the devastating consequences of major limb loss. Worldwide, more than 1 million extremity amputations are performed every year. Major lower extremity amputation is more commonly performed than major upper extremity amputation and indications for major limb amputation include vascular disease (54%), trauma (45%), and cancer (<2%). In elderly patients, the indication for major limb amputation is commonly vascular disease relating to peripheral vascular occlusive disease or diabetes. However, 80% of major amputations after severe limb trauma occur before the age of 40. Combat-related major limb amputations occur primarily in younger individuals and lead to long-term functional and psychosocial deficits. For example, during the recent American military operations in the Middle East, nearly 10% of the medically evacuated personnel suffered from injuries that resulted in major limb amputation. Regardless of the reason for major limb loss, all patients sustaining limb loss will contend with postamputation pain and a considerable number of them will develop debilitating chronic pain.
Postamputation pain is a general term that encompasses various unpleasant sensations experienced by patients with limb loss after the acute postoperative period. Postamputation pain can affect up to 95% of all patients with limb loss and is generally categorized into several categories: (1) phantom sensations, (2) phantom limb pain, and (3) residual limb pain. Despite being commonly designated as a type of postamputation pain, phantom sensations are nonpainful perceptions of their absent limb that can be kinetic (ie, perceived movements of the amputated part), kinesthetic (ie, perceived shape or position of the amputated part), or exteroceptive (ie, perceived touch, pressure, itching, vibration, etc, in the phantom limb). Studies demonstrate that up to 90% of patients will experience some sort of phantom sensation within 6 months of amputation. Distinct from phantom sensations, phantom limb pain is a pathologic, painful condition impacting the phantom limb. , , Phantom limb pain is thought to be due to functional reorganization of the somatosensory cortex, leading to chronic perceptions of pain in the portion of the limb that has been amputated. , Studies demonstrate that up to 85% of patients with limb loss suffer from phantom limb pain. ,
In contrast with the phenomena of phantom sensations and phantom limb pain, both mediated by mechanisms involving the central nervous system, residual limb pain (also known as stump pain) is defined as pain that is localized within the residual limb and caused by a variety of local factors, such as peripheral nerve neuromas, wounds, heterotopic ossification, osteophytes, bursa, and poorly fitting prosthetic devices. , , , Symptomatic neuroma formation can be common in patients with limb loss, and existing strategies for addressing this condition are largely ineffective. This article focuses on a novel surgical technique that uses regenerative peripheral nerve interfaces (RPNIs) to treat and prevent neuroma formation. Additionally, this review discusses the potential for RPNI surgery to modulate the experience of phantom limb pain in patients with limb loss.
Symptomatic neuromas
A transected peripheral nerve will attempt to regenerate to reestablish contact with motor or sensory end organs; however, in the setting of amputation, the regenerating axons are prone to forming an end neuroma consisting of a painful mass of axonal sprouts, connective tissue, and blood vessels. Ectopic activity, mechanical sensitivity, and chemosensitivity to catecholamines within the microenvironment of the neuroma creates discomfort for the patient. Within a neuroma, there is altered expression of transduction molecules, upregulation of sodium channels, downregulation of potassium channels, and development of nonfunctional connections between axons. This series of unfavorable events exacerbates neural hyperexcitability and spontaneous discharge seen within symptomatic painful neuromas. Although every transected peripheral nerve is expected to form a terminal neuroma, 50% to 70% of these neuromas will be symptomatic for patients with limb loss. Symptomatic neuromas are frequently the cause of residual limb pain and are known to compromise prosthetic rehabilitation and diminish the quality of life after amputation. In fact, painful neuromas have been cited to be a key factor driving patient abandonment of a prosthetic limb in up to 30% of cases.
Although neuroma pain is a separate entity from phantom limb pain, a growing body of evidence demonstrates that pain derived from the peripheral nervous system influences central nervous system pain mechanisms and vice versa. Specifically, ongoing neuropathic pain originating from symptomatic neuromas can lead to imbalances between excitatory and inhibitory signaling in the central nervous system, a phenomenon known as central sensitization. Alterations in ion channel function in sensory fibers (such as Aβ, Aδ, and C) in the peripheral nervous system affect spinal cord activity, leading to an excess of excitation and loss of inhibition. Perceptions of pain then travel via the spinothalamic pathway and are projected to the somatosensory cortex, where the location and intensity of the pain is registered. Eventually, this feedback of chronic nociceptive input results in a variety of maladaptive functional changes to the central nervous system. Ultimately, these changes contribute to a vicious cycle where central sensitization stemming from peripheral pain then further amplifies the experience of peripheral pain. Accordingly, in the setting of amputation, effective treatment of symptomatic neuromas will not only alleviate pain within the residual limb, but also interrupt the pathway of noxious afferent signals sensitizing central structures and furthermore may modulate centrally mediated pain, such as phantom limb pain. ,
Surgical Treatment of Symptomatic Neuromas
Although many treatment modalities have been explored for symptomatic neuromas, none are universally accepted. Nonsurgical treatment attempts have included desensitization, work hardening, biofeedback, chemical or anesthetic injections, transcutaneous electrical nerve stimulation, topical lidocaine, pain catheters administering local anesthetics, and medications (antidepressants, anticonvulsants, and opioids). However, symptomatic neuromas are most definitively treated with surgical techniques. A recent meta-analysis suggests that surgical treatment of symptomatic neuromas can be effective in more than 75% of patients and significantly improves patient-reported outcomes such as pain, depression, and quality of life. , Many surgical treatments for symptomatic neuromas have been described, such as simple neuroma excision alone ; nerve capping ; excision with transposition into bone, vein, or muscle , ; and nerve grafting. Although surgery has been shown to be beneficial in treating symptomatic neuroma, there is no consensus on which technique is superior for long-term outcomes.
The most common technique for the treatment of a symptomatic end neuroma involves resection of the terminal bulb and implantation of the residual peripheral nerve into a nearby muscle belly. On the basis of neurophysiology, the freshly cut peripheral nerve will undergo a process of axonal sprouting and elongation; however, this process will undoubtedly lead to a recurrence of the neuroma because the muscle belly is already fully innervated by its native motor nerve and thus the regenerating axons of the transected peripheral nerve will not be presented with any targets to reinnervate. Nonetheless, the recurrent neuroma will be cushioned within the recipient muscle belly and better protected from external stimuli such as pressure from the socket of a prosthetic device. Therefore, any alleviation of pain from application of this strategy is likely the result of simple transposition of the recurrent neuroma to a somewhat more favorable location. For this reason, a superior surgical strategy to treat symptomatic neuromas would capitalize on the physiologic process of nerve regeneration to actually prevent the reformation of a neuroma. In turn, such a technique would also serve to diminish the noxious afferent feedback contributing to central sensitization and possibly mitigate the experience of phantom limb pain.
The Regenerative Peripheral Nerve Interface
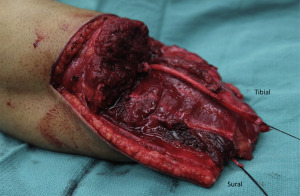
The RPNI is a simple and reproducible surgical solution to prevent neuroma formation that leverages several biologic processes and addresses many of the limitations of existing neuroma treatment strategies. An RPNI is constructed by implanting the distal end of a transected peripheral nerve into a free skeletal muscle graft ( Figs. 1–3 ). Because the denervated muscle graft is initially devascularized at the time of harvest, it will undergo a well-described process of degeneration followed by regeneration. , Early in this process, the graft is supported by imbibition; however, after regeneration the muscle graft becomes revascularized just as any free tissue graft would be after nonvascularized autologous transfer (ie, a full-thickness or split thickness skin graft). These regenerating muscle fibers concomitantly serve as denervated targets for regenerating axons sprouting from the end of the peripheral nerve. As a result of these functional connections, substantially fewer regenerating axons will be available to form a problematic neuroma. Through the processes of muscle revascularization and regeneration, and nerve regeneration and reinnervation, with formation of new neuromuscular junctions within the muscle graft (synaptogensis), a mature RPNI is a stable biologic structure that naturally minimizes neuroma formation. The RPNI was originally conceived of as a means to transduce and amplify neural signals for the purpose of controlling a neuroprosthetic limb. Many preliminary studies regarding RPNI use in prosthetic control in animal models and human subjects have also demonstrated the biologic stability, longevity, and functional capabilities of RPNI and its potential to reduce neuroma formation. ,
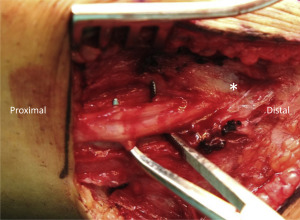
Synaptogenesis Mitigates Neuroma Formation
After peripheral nerve transection injury, the nerve undergoes 3 biologic processes: Wallerian degeneration, axonal sprouting/regeneration, and muscle reinnervation if end organs are present. When the distal end of a nerve is in proximity to the proximal end of the nerve (ie, end-to-end neurorrhaphy) axons will regenerate into distal endoneurial tubes and elongate until they reinnervate the end organ, achieving maximal functional recovery. However, in the context of amputation, the end organs are missing and therefore all of the peripheral nerves in the residual limb continue to sprout and regenerate until they form a neuroma. The use of free muscle grafts during RPNI surgery offers of a vast supply of denervated muscle targets for the regenerating axons. This distinguishing characteristic of RPNI surgery wholly differentiates it from the conventional technique of implanting a peripheral nerve into an already innervated local muscle belly. For regenerating efferent motor neurons, RPNIs provide ample denervated muscle fibers to facilitate the reestablishment of neuromuscular junctions. Denervated muscle fibers atrophy unless axonal input is reinstated within a finite period of time. In response to loss of innervation, these muscle fibers display dispersion of acetylcholine receptors along the sarcolemma and express of a variety of neurotrophic factors, events that foster an environment conducive to reinnervation. A regenerating axon that then makes contact with a denervated muscle fiber provides necessary trophic support to prevent atrophy, induces congregation of acetylcholine receptors to reform a neuromuscular junction, and initiates reconstitution of a motor unit. This innately orchestrated process of reinnervation has been observed with the RPNI. Early immunohistochemical studies in animal models revealed extensive colocalization of motor end plates and terminal axons within the free muscle graft of an RPNI. Electrophysiologic experiments confirmed that these new neuromuscular junctions were functional and capable of transducing nerve signals to muscle action potentials. More recent studies investigating the potential of RPNIs to facilitate control of an artificial limb in human subjects have also corroborated the fact that functional neuromuscular junctions develop within RPNIs. , These findings are compelling evidence that RPNIs can reduce neuroma formation in transected peripheral nerves containing motor axons by giving these axons targets to form neuromuscular junctions. As a result of this extensive research, RPNIs have been increasingly performed in human patients both to treat symptomatic neuromas and to prevent neuromas at the time of amputation.
Although efferent motor neurons can be expected to form new neuromuscular junctions within RPNIs, the fate of regenerating afferent sensory axons (eg, digital nerves) within a free muscle graft remain unclear. However, preliminary clinical experience suggests that RPNIs may be quite effective in preventing neuroma formation in sensory nerves as well. Although the exact mechanism of how free muscle grafts mitigate neuroma pain stemming from regenerating sensory axons is currently under investigation, studies that have investigated the possibility of sensory-to-motor crossover provide potential explanations for this effect. Previous studies have demonstrated the ability of sensory axons to provide trophic support of denervated muscle tissue, a phenomenon known as sensory protection. In these experiments, a donor sensory nerve is coapted to a motor nerve after denervation of a skeletal muscle. In this case, the sensory protected skeletal muscle demonstrates significantly preserved structure and function compared with controls. Notably, although neuroma formation was not a primary end point in these studies, none of these reports include a description of neuromas in the histologic or immunohistochemical analyses. Therefore, it is probable that, when regenerating sensory axons reach the denervated muscle distal to the site of nerve coaptation, neuroma formation does not occur as a result of the neurotrophic and myotrophic milieu characteristic of sensory protection. Furthermore, there is strong evidence that, although functional neuromuscular junctions may not be expected to form, sensory receptors (Golgi tendon organs and spindle cells) within the skeletal muscle can be readily reinnervated by donor sensory axons. These collective findings, along with preliminary experience with sensory protection in the clinical setting, , suggest that, when a donor sensory nerve is introduced to a denervated muscle, the regenerating axons are engaged in physiologically trophic processes that in turn diminishes the number of axons available for symptomatic neuroma formation. Based on this premise, symptomatic neuromas may be treated or even prevented by implanting a transected sensory nerve into a free muscle graft to create an RPNI.
Outcomes of Regenerative Peripheral Nerve Interface Surgery to Treat and Prevent Neuroma Pain
Studies examining the efficacy of RPNI surgery to treat symptomatic neuromas were inspired by enormously positive feedback from patients and their physiatrists during the course of rehabilitation after limb amputation. Among patients with limb loss who underwent RPNI surgery for the purpose of treating symptomatic neuromas, 71% of patients reported a decrease in neuroma pain within the residual limb. Remarkably, there was also a 53% decrease in phantom limb pain, a finding that supports the concept of peripheral nerve treatments favorably influencing centrally mediated pain. These patients also reported significantly decreased pain interference, high satisfaction levels, and more than one-half were using fewer pain medications after RPNI surgery. Additionally, more patients were also able to use their prosthetic limb after RPNI surgery, indicating that improved management of postamputation pain also translates to better function. These patient-reported outcomes highlight the potential that RPNIs have in treating symptomatic neuromas in amputation patients.
Symptomatic neuromas and phantom limb pain can also be mitigated by performing RPNI surgery at the time of limb amputation. A recent retrospective investigation compared postamputation pain outcomes between patients who underwent RPNI surgery prophylactically at the time of amputation and control patients who underwent standard limb amputation without RPNI surgery. Control patients were matched for age, gender, level of amputation, and mean duration of follow-up. RPNI patients experienced a substantially lower incidence of symptomatic neuromas within the residual limb compared with control patients (0% vs 13.3%). Moreover, at a mean follow-up time of almost 1 year, RPNI patients reported a significantly lower rate of phantom limb pain (51.1% vs 91.1%). Although the control group was overrepresented by vascular patients with ischemic disease, an examination of major and minor complications between groups suggests that the addition of RPNI surgery performed at the time of major limb amputation does not increase the overall complication rate. Given this encouraging preliminary experience, RPNI surgery is being performed prophylactically in a variety of clinical settings, such as hand and digit amputations, large soft tissue tumor resections, and major limb amputations at all levels, including hip and shoulder disarticulations. As the indications for RPNI surgery expand, ongoing prospective studies are being conducted to elucidate the efficacy of RPNI surgery to treat and prevent postamputation pain.
Regenerative Peripheral Nerve Interface Surgery and Targeted Muscle Reinnervation for Symptomatic Neuromas
Given the understanding that neuromas will form when regenerating axons are not presented with end organs for reinnervation, any strategy that reduces the number of aimless axons within a residual limb will serve to reduce symptomatic neuromas. In recent years, both RPNI surgery and targeted muscle reinnervation (TMR) have been developed as innovative methods to facilitate prosthetic control as well as neuroma prevention. TMR is a prosthetic control strategy that involves multiple nerve transfers to reroute transected peripheral nerves from an amputated limb to motor nerve branches of donor muscles. For example, in the setting of a transhumeral amputation, the median nerve can be coapted to small musculocutaneous nerve branches that innervate the short head of the biceps. After coaptation, axons from the median nerve will innervate a discrete portion of the biceps muscle, allowing this area of the muscle to generate electromyographic (EMG) signals representing median nerve information that can be recorded from a transcutaneous electrode. In regards to the potential of providing multiple independent signals for operating a prosthetic device with a high number of degrees of freedom, there are fundamental differences between RPNI surgery and TMR that are beyond the scope of this discussion. Regardless of these differences, like RPNI surgery, TMR may decrease neuroma formation by capitalizing on biologic processes characteristic of regenerating peripheral nerves. Both techniques involve reinnervation of denervated muscle and in doing so both strategies foster the formation of functional connections for regenerating axons. This in turn will minimize the number of axons available to contribute to symptomatic neuromas. As a method for managing postamputation pain, both RPNI surgery and TMR are increasingly performed in human patients to treat or prevent symptomatic neuromas.
However, it is important to note that with TMR there is an inherent need to sacrifice donor nerves and partially denervate muscles that might otherwise still be useful during prosthetic rehabilitation, such as with a myoelectric prosthesis. Myoelectric artificial limbs are commercially available devices that detect EMG signals produced by residual muscles in the residual limb. For example, EMG signals from the biceps and triceps muscles in a transhumeral amputation patient could be harnessed by transcutaneous electrodes to control the opening and closing of a myoelectric robotic hand. It is, therefore, possible that partial denervation of these residual muscles for TMR may complicate the acquisition of discrete high-fidelity EMG signals and ultimately compromise the reliability of myoelectric device use. Another important consideration is that the nerve transfers in TMR usually involve very large size mismatches at the site of nerve coaptation. Although the discrepancies in nerve sizes between the donor nerves and the recipient nerves used for TMR have not been quantified, in some cases the axon count for the donor nerve (eg, median nerve at the level of the upper arm) may approach an order of magnitude higher than the axon counts of the recipient motor branches (eg, branches to the short head of the biceps). Size mismatch is a known risk factor for formation of a neuroma in continuity. Furthermore, the proximal end of the sacrificed motor nerve in TMR is shortened and relocated away from the coaptation site because otherwise it may reinnervate its native muscle and interfere with the nerve transfer. However, it is precisely this natural propensity for the donor nerve to regenerate and attempt reinnervation of its original muscle that conceivably could lead to neuroma formation of the sacrificed nerve. These additional neuromas resulting from TMR could potentially contribute to residual limb pain and central sensitization.
In contrast, RPNI surgery does not involve denervation of any residual muscles, leaving their EMG signals intact and fully accessible for capture of EMG signals to control a prosthetic device. RPNI surgery is efficient and does not require tedious dissection to isolate small motor branches to muscle within the residual limb. Furthermore, because no nerve transfers are involved and no intact native nerves are cut, there is not the potential of forming neuromas in continuity or end neuromas. Despite these differences, as continued research elucidates the merits of RPNI surgery and TMR, the 2 techniques may have complimentary roles depending on the rehabilitative goals of a patient with limb amputation. Ultimately, both RNPI surgery and TMR have been shown to decrease postamputation pain, and the mechanisms by which they do so are rooted in the fact that both methods leverage principles of neurobiology to minimize the number of aimless regenerating axons forming neuromas.
Indications for Regenerative Peripheral Nerve Interface Surgery for Symptomatic Neuromas
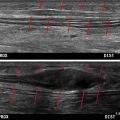
RPNI surgery is indicated for the treatment of all symptomatic neuromas and is commonly performed after major limb amputation. All patients who have had major limb amputation without any effort to provide new targets for reinnervation can be expected to have multiple neuromas, many of which will be symptomatic. Patients will report localized tenderness caused by a neuroma that is epitomized by neuropathic qualities, such as zinging, shooting, stabbing, or electrical shock pains. Usually, patients can point to the exact location where the neuroma pain originates. Tinel’s sign is positive when neuropathic pain is elicited by robust tapping precisely over the location of the symptomatic neuroma. The presence of a symptomatic neuroma can be confirmed by demonstrating alleviation of the pain after injection of local anesthetic solution into the area. Further confirmation and documentation of the neuroma can be achieved through ultrasound imaging. Ultrasound imaging may also be useful in diagnosing other symptomatic neuromas or other anomalies that may be contributing to residual limb pain. For example, if several symptomatic neuromas exist within the residual limb along with bone spurs or heterotopic ossification, treatment of just the most painful neuroma with RPNI surgery may not lead to a satisfactory decrease in residual limb pain. In these instances, patients may report alleviation of neuroma pain at one site, but will notice more predominant pain in the other areas owing to an unmasking effect of only removing the most noxious cause of pain. This unmasking effect has been demonstrated in other realms such as nerve compression surgery and migraine surgery. Notably, some neuromas identified by ultrasound imaging may not be symptomatic. RPNI surgery on nonpainful neuromas should be considered on a case-by-case basis. Although surgical intervention may reduce unfavorable neural feedback from the neuroma and help to diminish centrally mediated phenomena such as phantom limb pain, traumatic disruption of a nonsymptomatic neuroma may in itself cause additional pain.
In addition to a full workup of all potential causes of residual limb pain, preoperative consultation should involve a disclosure of any other painful conditions, such as chronic back pain, and longstanding pain medications. Both can affect central sensitization and therefore obfuscate the efficacy of RPNI surgery. Additionally, a thorough discussion of the risks and benefits of RPNI surgery must include a discussion regarding the donor site for muscle grafts. When RPNI surgery is performed after major upper or lower limb amputation, the most common donor site is the vastus lateralis through a separate thigh incision. Multiple small muscle grafts can be harvested without significantly affecting the function of the vastus lateralis muscle and the donor site morbidity is minimal. Alternative donor sites include distal residual muscles at the site of major limb amputation or muscle that are considered expendable depending on the level of amputation (eg, gracilis, brachioradialis). Another important goal of the preoperative visit is to set expectations about pain relief. Although RPNI surgery offers a physiologic means of decreasing neuroma pain by providing peripheral nerve axons targets for reinnervation, incomplete pain relief is a possibility. Although this may be due to untreated cases of pain within the residual limb, patients with chronic pain often experience remodeling of the central nervous system that impedes resolution of pain, even when the peripheral trigger is treated. For this reason, a multidisciplinary approach to pain control after RPNI surgery is recommended involving providers from various fields such as physical medicine and rehabilitation, physical therapy, occupational therapy, and primary care.
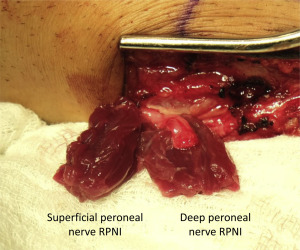
RPNI surgery can also be performed to prevent neuroma formation at the time of amputation ( Figs. 4–6 ). Free muscle grafts can be harvested to created RPNIs involving any transected peripheral nerves, including large mixed nerves (eg, sciatic nerve, tibial nerve, median nerve) and purely sensory nerves (eg, radial sensory nerve, digital nerve, intercostal nerve). The principal goal of performing RPNIs concurrently with amputation surgery is to minimize the formation of painful neuromas at the ends of transected peripheral nerves. In turn, this will decrease the need for pain medications postoperatively and facilitate prosthetic rehabilitation. Furthermore, prevention of peripheral nerve pain is likely to mitigate the experience of phantom limb pain and avert the maladaptive feedback to the central nervous system that is known to contribute to chronic pain syndromes.