5 Reconstructive surgery
Lower extremity coverage
Synopsis
 The reconstructive surgery for the lower extremity have evolved from a staged approach to proving best solutions for functional and cosmetic outcome.
The reconstructive surgery for the lower extremity have evolved from a staged approach to proving best solutions for functional and cosmetic outcome.
 This chapter covers the classical approach with a gradual change of principle that advocates an one stage elevator approach.
This chapter covers the classical approach with a gradual change of principle that advocates an one stage elevator approach.
 Special considerations should be given to overcome the complexity of lower extremity reconstruction, such as diabetes and chronic infection.
Special considerations should be given to overcome the complexity of lower extremity reconstruction, such as diabetes and chronic infection.
 Finally, introduction of perforator flaps, the use of multiple flaps by combination, and supermicrosurgery will help you design and widen the reconstructive choice for the lower extremity.
Finally, introduction of perforator flaps, the use of multiple flaps by combination, and supermicrosurgery will help you design and widen the reconstructive choice for the lower extremity.
Introduction
Although early amputation and prosthetic treatment was thought to offer the potential of faster recovery and lower cost, recently reports have provided different views. A multicenter, prospective, observational study to determine the functional outcome of 569 patients with severe leg injuries resulting in reconstruction or amputation has provided information based on Sickness Impact Profile, a measure of self-reported health status.1 Although reconstruction may be faced with more challenging process, the Lower Extremity Assessment Project, or LEAP study showed no significant difference in outcome at 2 years. Costs following amputation and salvage were also derived from data in a study that emerged from the Lower Extremity Assessment Project concluding that amputation is more expensive than salvage and amputation yields fewer life quality-adjusted life-years than salvage.2 Other reports have shown similar findings where projected lifetime healthcare cost for amputation may be as high as three times.3,4
History
The early history of lower extremity reconstruction dates back to Hippocrates (460–370 bc) and amputation and procedures trying to improve the success of amputation and survival has been the main practice up to the First World War.5
Although the term “flap” originated in the 16th century from the Dutch word flappe, meaning something hanging by one side, and the concept dates back as far as 600 bc when Sushruta Samhita described nasal reconstruction using a cheek flap, it was not until the First and Second World Wars that pedicled flaps were used extensively for reconstruction of the extremity. The next evolution came as surgeons started to use axial pattern flaps. The term “axial pattern” used to describe flaps with named pedicles was presented by McGregor and Morgan and this concept along with contributions from many surgeons led to the understanding of hemodynamic aspect of flap circulation.6–8 The first muscle flap for lower extremity coverage was first described by Stark for coverage of debridement sites for osteomyelitis but went unnoticed until Ger reported that the leg muscles were reliable for leg coverage with abundant blood supply.9,10 These progresses led to the distinction between axial and random flaps and muscle and musculocutaneous flaps which led to the introduction of free tissue transfer.
Prior to the introduction of microsurgery, limitations existed to reconstruct large and extensive wounds including bone defects. With the opening of the era of microsurgery, tissue coverage for extensive and complex defects, even after severe trauma and radical debridement, truly allowed advances in lower extremity coverage. In 1986, a landmark publication for reconstructive microsurgery in extremity was presented by Marko Godina, when he established the principle of early debridement, free tissue transfer, and aggressive rehabilitation to achieve functional limb after salvage.11 Based on this principle and advances made in the new millennium, treatment for infected wounds like osteomyelitis, complicated wounds such as diabetic foot ulcers and ischemic limb, and large defect after cancer ablation now turns routinely to soft tissue coverage using microsurgery. With respect to function, free functioning muscle transfer using rectus femoris muscle and gracilis muscle and composite free flaps such as dorsalis pedis with extensor tendons or fibula osteocutaneous flaps may achieve acceptable functional results in extremity with composite defects.12–15 Innervated flaps utilizing microsurgical coaptation of nerves may help regain early protective sensation for sole reconstruction.16–18 Tissue expansion technique combined with or without microsurgery may allow coverage for large chronic defect or healed scars minimizing donor morbidity.19 The most recent advances in flap surgery came in the 1990s, with the introduction of perforator flaps. Koshima and Soeda described the use of an inferior epigastric artery skin free flap without rectus abdominis muscle for reconstruction.20 This implied reduction of donor site morbidity, being able to be tailored regarding thickness, and increased freedom of orientation of pedicle allowing more flexibility while providing coverage of the defect.21,22 Advances in wound healing science have synergistically made closure of lower extremity wounds more successful. The vacuum-assisted closure may provide stable temporary dressing which allows increased blood flow and decreased bacterial counts to allow better environment for flap coverage.23,24
Principles
An evaluation of the patient as a whole allows proper decisions to be made within regards to systemic conditions, socioeconomic status, and rehabilitative potential. Extremity injuries are best approached by teams of surgeons with knowledge of skeletal, vascular, neurologic and soft tissue anatomy. Although evaluations such as Mangled Extremity Severity Score (MESS), the Predictive Salvage Index, and the Limb Salvage Index can assist the team in making a decision for amputation, it must not be used as a sole criterion and the decision to amputate must be individualized for each patient.19,25–28
The value of autologous tissue
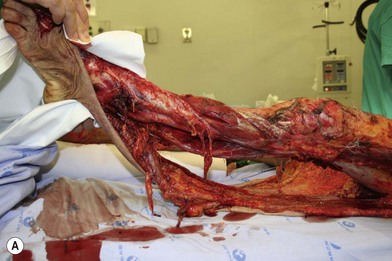
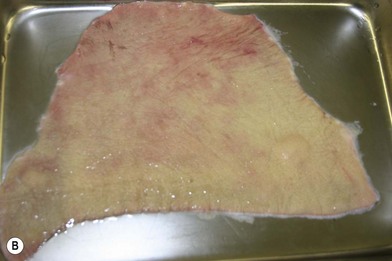
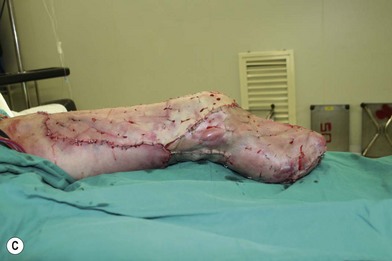
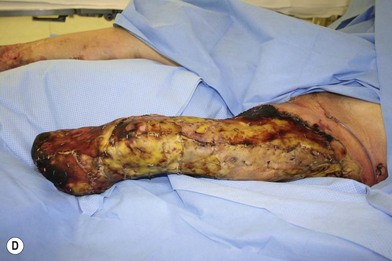
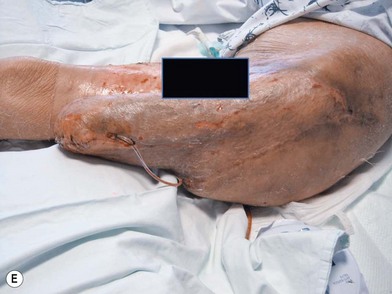
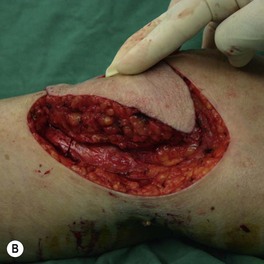
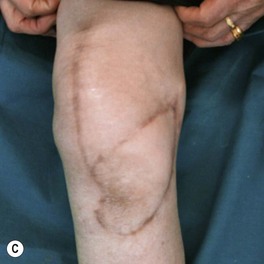
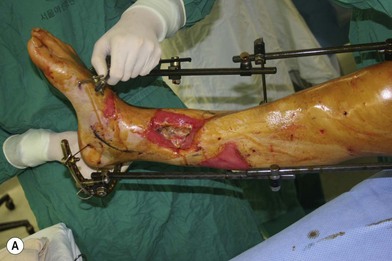
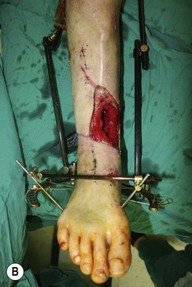
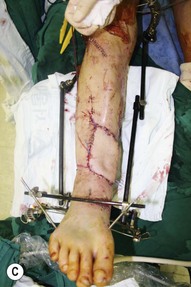
Whether acute or chronic, evaluation of lower extremity wounds and the eligibility for soft tissue reconstruction begins with vascular status evaluation. If clinical and diagnostic examination reveals inadequate perfusion and the value of reconstruction minimal, amputation should be individually decided. An amputated or avulsed tissue should never be disregarded, especially in acute traumas, unless severely contaminated or lacks vascular structure. Nothing can mimic the superiority of an autologous tissue and all tissues from amputated parts should be considered as potential donor tissues for reconstruction. The skin harvested from the degloved or amputated part can be utilized as biologic dressings to permanent skin grafts (Fig. 5.1).29 The leg length can be preserved using soft tissue distal form the zone of injury as fillet pedicled or free flaps.30–33 Amputated bones can be banked or used as a flap to reconstruct the leg.34,35
The reconstructive elevator
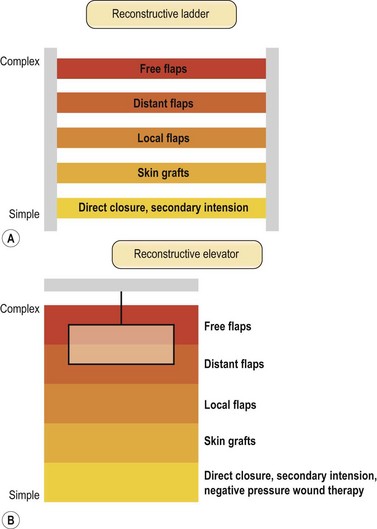
Once the wound is evaluated to have good vascular supply, stable skeletal structures and a relatively clean wound, soft tissue coverage is then considered. The concept of reconstructive ladder was proposed to achieve wounds with adequate closure using a stepladder approach from simple to complex procedures (Fig. 5.2A). Although still valued and widely taught, the reconstructive ladder comes from the concept of wound-closure ladder dating back beyond the era of modern reconstructive surgery.36 In the era of modern reconstructive surgery, one must consider not only adequate closures but form and function. A skin graft after mastectomy can still provide coverage but a pedicled TRAM (Transverse Rectus Abdominis muscle) flap will provide superior results in addition to coverage. Now with introduction of DIEP (Deep Inferior Epigastric Perforator) flaps, the reconstructive ladder approach seems to show more flaws. Other techniques including tissue expansion, skin stretching and vacuum-assisted closure has made new changes in approaching reconstructive options. A simpler reconstructive option may not necessarily produce optimal results. This is especially true for lower extremity coverage, where consequences of inadequate coverage will lead to complications such as additional soft tissue loss, osteomyelitis, functional loss, increased medical cost and even amputation. Thus to provide optimal form and function, we jump up and down the rungs of the ladder. The reconstructive elevator requires creative thoughts and considerations of multiple variables to achieve the best form and function rather than a sequential climb up the ladder (Fig. 5.2B). This paradigm of thought does not eliminate the concept of reconstructive ladder but replaces it as a ladder of wound closure and makes its mark in the field where variety of advanced reconstructive procedures and techniques are not readily available. Based on the reconstructive elevator, method of reconstruction should be chosen based on procedures that results in optimal function as well as appearance.
Skin grafts and substitutes
Autologous skin grafts are used in variety of clinical situations. It can be full or partial thickness and requires a recipient bed that is well vascularized and free of bacterial contamination. The split-thickness grafts are usually used as the first line of treatment where wounds cannot be closed primarily or undue tension is suspected. In the extremity often with complex wounds; bone exposure and/or avascular beds, infected wounds, wound with dead space and poorly coagulated beds, skin grafts should be avoid. Autologous cultured keratinocytes can be used where split-thickness donor sites are limited. However, the use of cultured epithelial autograft has been hampered by reports that show it to be more susceptible to bacterial contamination, has a variable take rate, and is costly.37
A skin substitute is defined as a naturally occurring or synthetic bioengineered product that is used to replace the skin in a temporary, semi-permanent or permanent fashion.38 Temporary epidermal replacements may be beneficial in superficial to mid-dermal depth wounds. In deeper wounds, dermal replacements are of primary importance. Bioengineered products for superficial wounds are porcine products such as EZ-derm and Mediskin (Brennen Medical-LLC, St Paul, MN), which helps to close the wound, decrease pain and improve rate of healing.38 Biobrane (UDL Laboratories Inc, Rockford, Illinois) is a bilaminate skin substitute that is used temporarily. The outer layer is formed with thin silicone payer with pores that allow removal of exudates and penetration of antibiotics. The inner layer is composed of three dimensional nylon filament weave impregnated with type I collagen to adhere to the wound. Bioengineered products that are used for deep wounds are Allograft, Alloderm (Life Cell Corporation, Woodlans, TX), Integra (Integra Life Sciences, Plainsboro, NJ), and Apligraf (Organogenesis Inc, Canton, MA). The gold standard for temporary skin coverage is cadaver skin or allograft. Allograft is used to cover extensive partial- and full-thickness wounds. It prevents tissue dessication, decreases pain, insensible loss of water, electrolytes and protein, suppress the proliferation of bacteria, and decreases the hypermetabolic component of thermal injuries.39,40 Alloderm is an acellular dermal matrix engineered from banked, human cadaver skin. It can also provide single stage reconstruction when used with a split-thickness skin graft.41 Alloderm is known to improve functional and cosmetic results in deep burn wounds (Fig. 5.3).42 Integra is an acellular collagen matrix composed of type I bovine collagen cross-linked with chondroitin-6-sulfate and covered by a thin silicone layer that serves as an epidermis.43 It is readily available and does not require a donor site and coverts open to closed wound and decreases metabolic demand on the patient. However, Integra must be used on clean wounds and requires a two-stage procedure later for the graft. Simultaneous use with negative wound pressure therapy may accelerate the vascularization. Apligraf is a bilaminate human epidermal and dermal analogue that can act as a permanent skin substitute. The epidermal layer is formed by human keratinocytes with a well differentiated stratum corneum. The dermal layer is formed with bovine type I collagen lattice impregnated with human fibroblasts from neonatal foreskin. Apligraf is not antigenic and the dermal layer incorporates into the wound bed. Apligraf has been shown to significantly decrease the time of venous ulcer healing compared to compression.44
Approach by location (local flaps)
Thigh
The proximal thigh wounds can result from various causes such as complications from hip fractures, infected bypass vascular graft, after tumor resection, and trauma. The medial portion of the proximal thigh can be especially challenging due to location of vital structures and the likely formation of dead space. Local lower extremity muscle or myocutaneous flap options include using the flaps based from the lateral circumflex femoral artery such as tensor fascia lata, vastus lateralis, and rectus femoris flaps. Vertical rectus abdominis muscle or myocutaneous flap using the deep inferior epigastric artery can allow stable coverage of the proximal thigh. The gracilis muscle or myocutaneous flap based on the medial femoral circumflex artery may lack muscle bulk but is a good option when the dead space is not extensive. Now with increased knowledge of perforator and perforator based flaps, basically any perforator can be chosen as a source of vascular supply to the skin flap and be rotated to cover a defect.45–47 When the use of local flaps is not feasible due to the complexity of the wound, free tissue transfer is indicated.
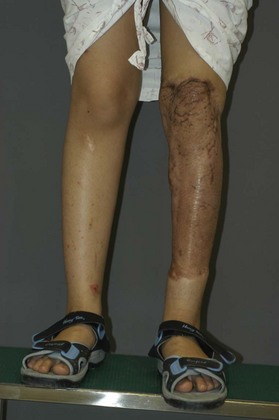
The wounds of the distal thigh (supracondylar knee) can be very difficult due to the limit of rotation from previously described local muscle or musculocutaneous flaps from the thigh. Pedicled medial gastrocnemius muscle or musculocutaneous flap from the lower leg can be extended to cover this region. However, extensive or complex defects may require free tissue transfer or coverage using a perforator based rotation/advancement skin flap (Fig. 5.4).
Microvascular free tissue transfer
Whichever flap you select, the guideline for lower extremity reconstruction using free flaps remains the same: anastomose the vessel outside the zone of injury, make end-to-side arterial anastomosis and end-to-side or end-to-end venous anastomosis, and reconstruct the soft tissues first and then restore the skeletal support.48
Treatment approach
Preoperative evaluation
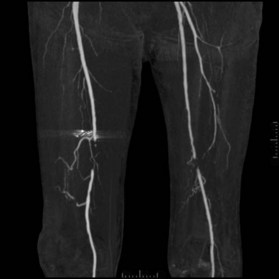
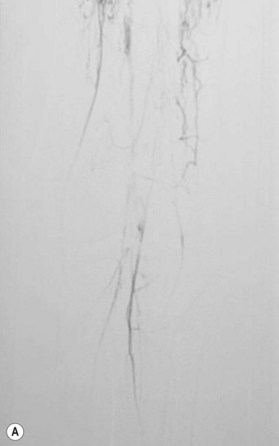
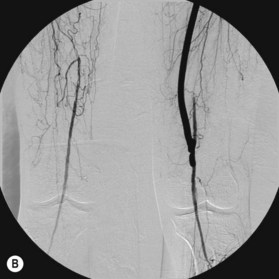
After the decision is made to reconstruct the lower extremity, the first preoperative evaluation should start with vascular status. Physical examination of palpable pulse, color, capillary refill, and turgor of the extremity allows to assess initial status and Doppler examination can provide additional information.49 The use of preoperative arteriography for lower extremity reconstruction is considered when physical/Doppler exam reveals inconclusive vascular status or chronic vascular disease is suspected (Fig. 5.5). The use of computed tomographic angiography may obtain vascular information of the recipient region without the risk of complications from arterial puncture of the groin and also can provide vascular information of the donor flap facilitating the planning and the surgical procedure.50–52 In association with prior injuries to the lower extremity, the routine preoperative use of angiogram is controversial.24,49,50,53–55 It is selectively recommended in patients who have loss of one or more peripheral pulses, a neurologic deficit secondary to the injury, or a compound fracture of the extremity that has undergone reduction and either external or internal fixation.54
Nerve injuries that are irreversible may require special considerations. Peroneal nerve injuries results in foot drop and loss of sensation of the dorsum of the foot. Thus lifelong splinting or tendon transfers may be required. Complete loss of tibial nerve function results in loss of plantar flexion and is a absolute contraindication for reconstruction.56 The loss of plantar sensation can be devastating and may hinder the need for reconstruction although is not an absolute contraindication.57
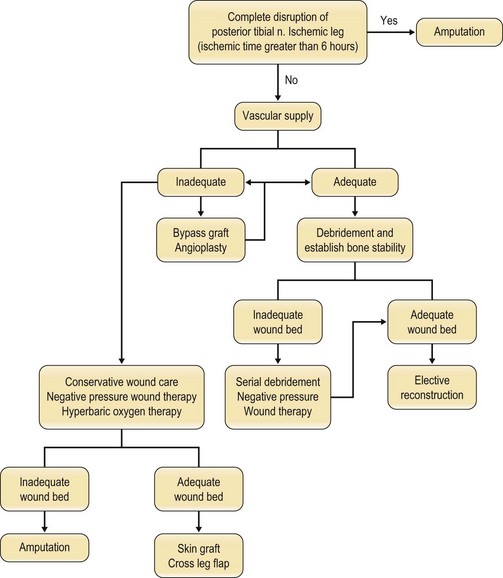
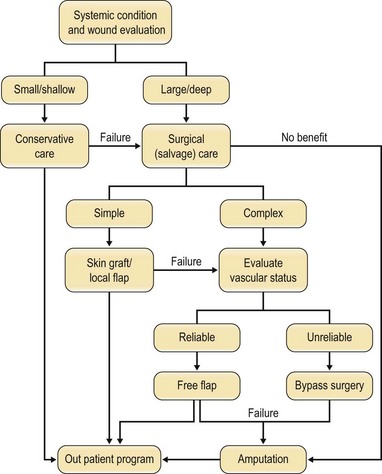
An algorithm of approach is outlined in Figure 5.6.
Primary limb amputation
A study by Lange describes absolute and relative indications for primary amputation of limbs with open tibial fractures.56 Absolute indications include: anatomically complete disruption of the posterior tibial nerve in adults and crush injuries with warm ischemia time greater than 6 h. Relative indications include: serious associated polytrauma, severe ipsilateral foot trauma, and anticipated protracted course to obtain soft tissue coverage and tibial reconstruction.
In these cases where limb salvage is not possible, attempts should be made to salvage as much limb length as possible. Every effort should be made to save the functional knee joint as below-knee amputation results in far superior ambulatory outcome and up to 2–3 folds more full mobility compared to above knee amputation.58 The energy consumption is far less for below-knee amputation and this allows these patients to walk significant daily distances, thus maintaining good quality of life.59 Though the ideal stump length below the knee is more than 6 cm, any length of tibia should be preserved.60 If adequate soft tissue exists, stump may be closed primarily and where local tissue is inadequate, microsurgery allows preserving maximal length of the stump. If the tissue distal to the amputation is usable, a fillet flap can be performed. Other flaps such as muscle, musculocutaneous, fasciocutaneous and perforator flaps can be used for microsurgical reconstruction and achieves the same goal as well. Muscle flaps may have a tendency to heal slowly and to shrink due to muscle atrophy, while skin flaps may provide better contour and sensibility.61
Debridement
The vacuum-assisted closure can be used to optimize the wound bed and minimize dressing changes until definitive reconstruction. It must be used with caution and in conjunction with serial debridement. It does not replace surgical debridement and should not be used in heavily contaminated wound with necrotic tissues. If a lower extremity wound is clean, bony stability is present, and no vital structures are exposed, application may be indicated.54 This device facilitates dressing of the wound and often promotes healing.
Timing of reconstruction
Regardless of the degree of contamination and extent of injury when indicated for salvage, there is no need to delay definitive coverage provided that the general condition of the patient and the status of the wound allows it. General consensus favors early aggressive wound debridement and soft issue coverage. Byrd et al. described acute, subacute, and chronic phases of an open tibial fracture.62 Ideally, the wound is covered in the first 5–6 days after injury at the acute phase of the wound. In severe Gustillo type IIIB and type IIIC injuries, free muscle transplantation obtained the best results. At 1–6 weeks the wound enters the subacute phase where wounds had higher tendency of infections and flap failures. Between 4 and 6 weeks, the wound enters the chronic phase and clear demarcation between viable and nonviable bone becomes apparent. Godina further demonstrated that radical debridement and coverage within 72 h results in best outcome where only 0.75% of flap fails, 1.5% are infected, and 6.8 months are needed for union of the bone.11 The failure rate compared remarkable to 12% when reconstructed from day 3 to 3 months and 10% when reconstructed after 3 months of injury. Yaremchuk et al. recommended early coverage between days 7 and 14 after several debridements allowing better identification of zone of injury.63 The common idea behind early intervention is that it minimizes the risk for increasing bacterial colonization and inflammation leading to complications. Acute coverage by day 5–7 is generally accepted as having a good prognosis in terms of decreased risk of infection, flap survival, and fracture healing.11,24,62,63 If patient condition does not allow prolong surgical procedures, then the wound should be debrided as early as possible and maintain a clean and well vascularized recipient bed till conditions allow definitive reconstruction.64
Selection of recipient vessel
Many lower extremity wounds resulting from trauma are high-energy injuries with a substantial “zone of injury.” This thrombogenic zone is known to extend beyond what is macroscopically evident, and failure to recognize the true extent of this zone is cited as a leading cause of microsurgical anastomotic failure. Within the zone, perivascular changes such as increased friability of vessels and increased perivascular scar tissue may lead to difficult dissection of recipient vessels and higher incidence of thrombosis after anastomosis.65 How extensive is clinically very difficult to realize. Thus, Isenberg and Sherman demonstrated that clinical presentation of recipient vessel (vessel wall pliability and the quality of blood from transected end of vessel) was more important than the distance from the wound.66 Park et al. also concluded that site of injury and vascular status of the lower extremity was the most important factor in choosing a recipient vessel.67 This idea was further supported by successful anastomosis of perforator to perforator adjacent to or within zone of injury.68 Based on these findings, one of the most important factors in selecting the recipient vessel may be the vascular quality itself.
Special considerations
Osteomyelitis
Osteomyelitis often follows severe open leg fractures with massive contamination or devascularized soft tissue and bone. Inadequate debridement or delayed coverage of the wound increases the chance for osteomyelitis and early debridement remains to be the key to prevention.69 Osteomyelitis should be seen as a spectrum of disease and should be individualized and managed accordingly. Factors known to be of prognostic significance include the duration of infection, the extent of bony involvement, the presence of associated fracture or nonunion, and overall immune status of the patient.70 The wound is composed of exposed bone, infected bone, devitalized bone, and scarred tissue surrounding the bone. These components have diminished vascular supply making antibiotics difficult to reach. Thus, to achieve the goal of infection control and the restoration of function, treatment principles for chronic osteomyelitis are debridement including the complete resection of involved bone, flap coverage with vascularized tissue, and brief course of antibiotic treatment (Fig. 5.7). Local method of antibiotic delivery can be used when complete debridement of bone is not possible. Although there have been controversy in selecting the type of flap for coverage, muscle have shown experimentally to have increased blood flow and antibiotics delivery, increased oxygen tension, increased phagocytic activity, and decreased bacterial counts in wounds reconstructed with muscle flaps than fasciocutaneous flaps.71–73 Clinically, complete debridement and obliteration of dead space are the most important steps to treat osteomyelitis and the type of flaps seems less crucial.74,75 Bone defects can be managed with vascularized bone flap, secondary bone grafting, bone distraction lengthening or a combination of these techniques.
Diabetes
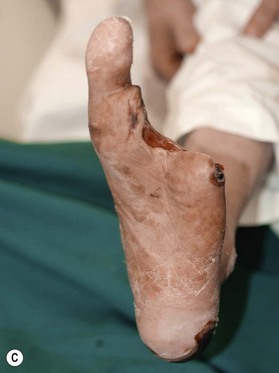
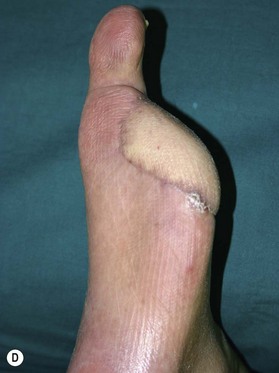
Patients with diabetes require additional concerns from chronic renal failures, nutrition to blood sugar control. These multiple issues are best approached by a multidisciplinary team.76–79 Patients will frequently have chronic bacterial colonization, osteomyelitis, complex wounds, bone deformity, local wound ischemia and vascular disease. The etiology behind these complicated wounds is from macrovascular angiopathy, bony deformities leading to pressure points, neuropathy, and poor metabolic control. When patients with diabetes are required to undergo reconstructive procedure of the extremity, vascular status must be evaluated to ensure success.80,81 Any vascular problems must be addressed first and corrected. If not correctable, the surgeon may be faced with a high risk of failure. One must consider the probability of successful reconstruction, based on eliminating the underlying problems of the diabetic wound and also take into account long-term ambulation after reconstruction. In a retrospective study by Hong, only 71 patients out of 216 were deemed functionally salvageable using microsurgical approach and reported successful outcome in 66 patients (Figs 5.8, 5.9).80 Large and composite diabetic wounds must be aggressively debrided including the necrotic bone and be covered with well-vascularized tissue.
Coverage after tumor ablation
As with any reconstructive procedure, the aim of reconstruction after tumor ablation is to maintain quality of life by preserving function and achieving acceptable appearance. In addition, coverage must be able to withstand adjuvant therapy with radiation therapy and/or chemotherapy and play a role to achieve long-term local control of disease. Surgeons should have close cooperation with the oncologists and must acquire adequate knowledge of tumor characteristics, behavior, and adjuvant treatment to plan and choose the type of reconstructive procedure. Knowing that reconstruction is feasible allows the oncologic surgeon to achieve a comfortable and satisfactory excision margin and may lead to a better outcome. Skin grafts are always an option especially for very extensive defects where flap coverage is not available. But for wounds scheduled for postoperative radiation therapy or located over joints and high friction regions, skin graft should be avoided and be reconstructed with a durable flap.82 Special consideration should be made to preoperative radiation therapy where skin would become fibrotic and ischemic around the cancer and thus will not allow local coverage. Regarding adjuvant chemotherapy, free flap procedures will not interfere with chemotherapy nor will chemotherapy have an impact on free flap survival.83,84 Overall success rate was shown to be 96.6% in a series of 59 free flaps in 57 patients undergoing lower extremity reconstruction after tumor ablation, with 12% major and 7% minor complications.83 Various flaps from omentum, muscle with skin graft, musculocutaneous, and perforator flaps can be used for reconstruction depending on location, size, depth, adjuvant therapy, function, and cosmetic appearance (Fig. 5.10).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree