Reconstruction of the Scalp, Calvarium, and Forehead
J. Guilherme Christiano
Nicholas Bastidas
Howard N. Langstein
Scalp and forehead defects result from trauma, burns, oncologic resection, infection, radionecrosis, and congenital abnormalities. Reconstruction is dictated primarily by the size and depth of the defect and is accomplished by the simplest means possible following the reconstructive ladder. Nevertheless, complicating features of the soft tissues and underlying bone may require a more complex approach.
ANATOMY
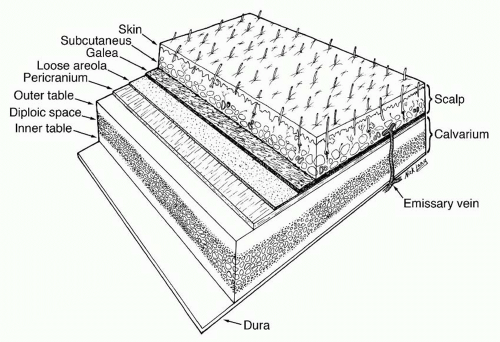
The forehead and scalp share five distinct anatomic layers: Skin, subcutaneous Connective tissue, galea Aponeurotica or muscle, Loose areolar tissue, and Pericranium (SCALP) (Figure 31.1). The first three layers are bound together by numerous vertical septae between the skin and the galea aponeurotica, forming a single unit that glides along the loose areolar tissue over the pericranium.
The skin of the scalp is the thickest in the body (between 3 and 8 mm) and has numerous sebaceous glands. Immediately beneath the skin lies a layer of dense connective and fatty tissue, which contains a rich net of arteries, veins, lymphatics, and sensory nerves, along with the hair follicles of the scalp. The underlying galea aponeurotica is part of a broad fibromuscular layer that covers the upper cranium from the forehead to the occiput and serves as the central tendinous confluence of the occipitalis muscle posteriorly and the frontalis muscle anteriorly. The occipitalis and frontalis muscles are thin, quadrilateral muscles, each consisting of two bellies joined in the midline by extensions of the galea. The occipitalis muscle arises from the lateral two thirds of the superior nuchal line of the occipital bone and from the mastoid part of the temporal bone. The frontalis muscle has no bony attachments. Its medial fibers are continuous with those of the procerus muscles, while its lateral fibers blend with those of the corrugator and the orbicularis oculi. The frontalis muscle joins the galea aponeurotica in the upper forehead. The galea aponeurotica is contiguous with the temporoparietal fascia (also known as superficial temporal fascia) and with the subcutaneous musculoaponeurotic system (SMAS) of the face (see Chapter 47).
Deep to the galea lies the loose areolar layer, a relatively avascular plane also known as the subaponeurotic layer, subgaleal fascia, or innominate fascia. It enables the layers above it (skin, subcutaneous connective tissue, and galea) to slide as a unit over the cranium. As such, this layer is easily dissected and is often the plane of cleavage in avulsion or scalping injuries.
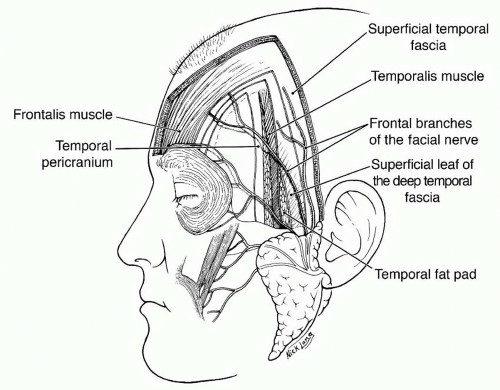
The pericranium is the periosteum of the calvarium. Laterally, at the superior temporal line, it is continuous with the deep temporal fascia (temporalis muscle fascia). More inferiorly, the deep temporal fascia divides into two layers, deep and superficial, which envelop the superficial temporal fat pad and insert into the superficial and deep aspects of the zygomatic arch, respectively (Figure 31.2).1
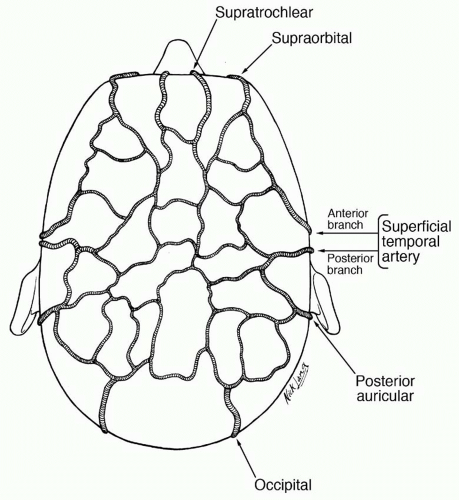
Blood Supply
The scalp and forehead are supplied by five paired arteries that form rich interconnections within the subcutaneous layer (Figure 31.3). From anterior to posterior, these are the supratrochlear and supraorbital arteries (internal carotid), and the superficial temporal, posterior auricular, and occipital arteries (external carotid).
The main blood supply to the anterior scalp and forehead derives from the supratrochlear and supraorbital arteries, which arise from the ophthalmic artery (first branch of the internal carotid) and enter the forehead vertically at the level of the supraorbital rim. These vessels become superficial above the brow, piercing the frontalis muscle to reach the superficial layer, where they anastomose with anterior branches of the superficial temporal arteries.
As terminal branches of the external carotid arteries, the superficial temporal arteries are the largest of the scalp vessels and supply blood to the temporal and central scalp. They course through the superficial lobes of the parotid glands and ascend in front of the auricles, traveling with the auriculotemporal nerves. Above the zygomatic arch, the superficial temporal arteries lie within the superficial temporal fascia and divide into anterior and posterior branches. These branches anastomose liberally with anterior and posterior blood supplies of the scalp. The anterior branch usually crosses the most anterior temporal branch of the facial nerve just above the lateral brow, an important anatomic landmark for finding either the nerve or the vessel.
The occipital arteries provide blood supply to the posterior scalp above the nuchal line. They run from the external carotid arteries along the vertebral muscles and join the scalp through the cranial attachments of the trapezius muscle or in the space between the trapezius and sternocleidomastoid muscles. These arteries are usually found within 2 cm of the midline at the nuchal line and usually divide into medial and lateral branches above this location. The blood supply of the posterior scalp caudal to the nuchal line derives from perforating branches of the trapezius and splenius capitis muscles.
The region of the mastoid is supplied by the posterior auricular arteries, the smallest of the main vessels of the scalp. They are sufficient for some local flaps in this vicinity but not robust enough to support the entire scalp.
The scalp is drained by veins that accompany the named arteries. Venous blood also flows through the diploe of the cranium to the dural sinuses via emissary veins. These emissary veins do not have valves and can be a portal for intracranial spread of infections in the scalp, especially those in the loose areolar layer.2 The supratrochlear and supraorbital veins join to form the angular vein on each side. They also communicate with the ophthalmic veins. The superficial temporal veins descend in front of the auricle and enter the parotid glands to join the maxillary veins and form the retromandibular veins. The posterior auricular veins join the posterior division of the retromandibular veins to form the external jugular veins. The occipital veins pierce the cranial attachments of the trapezius muscle and join the deep cervical and vertebral veins in the venous plexus of the posterior triangle on each side.
The scalp lymphatics are mainly located in the subdermal and subcutaneous levels. There are no lymph nodes in the scalp region and hence no barriers to lymphatic flow. Lymph from the scalp drains freely toward the parotid, pre- and postauricular nodes, upper cervical nodes, and occipital nodes.
Innervation
The muscles of the forehead are innervated by the frontal (also known as temporal) branches of the facial nerve (cranial nerve VII). As many as five separate branches may course in the loose areolar plane below the SMAS, cross the midportion of the zygomatic arch, and reach the undersurface of the frontalis muscle (Figure 31.2). The occipitalis muscle is innervated by the posterior auricular branches of the facial nerve. The temporalis muscle is supplied by motor branches from the third division of the trigeminal nerve (cranial nerve V).
The sensory nerve supply to the anterior scalp and forehead derives from the ophthalmic division of the trigeminal nerves. The supratrochlear and supraorbital nerves arise from this branch and leave the skull through the supraorbital foramina or grooves at the supraorbital rim. The temporal scalp is supplied by the maxillary division of the trigeminal nerve (zygomaticotemporal nerve) and the preauricular scalp by the mandibular division (auriculotemporal nerves). The postauricular scalp is supplied by dorsal rami of the cervical spinal nerves (greater occipital nerve and third occipital nerve).
SCALP RECONSTRUCTION
Several variables need to be taken into account when devising the best therapeutic approach for a scalp defect. While size and depth are the most obvious, other features may prove to be just as important in each individual case. As always, the plastic surgeon should employ the procedure at the lowest level of the reconstructive ladder that suits both the defect and the patient.
Key Planning Points
1. The Defect
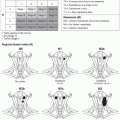
Size and shape along with depth (see below) are the main determinants of the amount of tissue needed for closure. Categorizing scalp defects by their size can facilitate an algorithmic approach to reconstruction. However, defects come in different shapes, and a 2 × 10 cm defect may represent a completely different reconstructive challenge compared with a 4 × 5 cm wound, even though both span 20 cm2. For this reason, while recognizing that most scalp wounds in the real world are not perfectly round, the authors choose to categorize the size of the defects based on diameter, as depicted below.
Location. The parietal regions allow the most advancement of scalp tissue. Therefore, parietal defects are more amenable to scalp undermining and primary closure, while defects in other areas may take advantage of full advancement of parietal scalp as a flap. Reconstruction of the temporal scalp may benefit from the extra soft tissue padding provided by the underlying temporalis muscle. The occipital region provides very limited scalp mobility. Finally, for best cosmetic results, defects in hair-bearing areas should be covered using hair-bearing scalp, unless hair transplantation is planned.
Depth. Lacerations involving the galea require specific closure of this layer. When skin grafting over the calvarium, an intact pericranium is needed for reliable and durable take. In defects involving the calvaria, all exposed devitalized bone needs to be debrided before coverage. Exposed dura frequently requires some form of calvarial reconstruction for brain protection, but small areas may be resurfaced with wellvascularized scalp or other soft tissue.
2. The Surrounding Tissues
Quality. Thickness, elasticity, and vascular supply to the tissues surrounding the defect can be compromised by several factors. In addition, scars from previous operations are
frequently seen in the scalp in patients referred for oncological reconstruction, further limiting the usefulness of local tissues. Burns and previous radiation therapy also diminish the use of local flaps. Infections should be controlled before the creation of flaps or insertion of tissue expanders.
frequently seen in the scalp in patients referred for oncological reconstruction, further limiting the usefulness of local tissues. Burns and previous radiation therapy also diminish the use of local flaps. Infections should be controlled before the creation of flaps or insertion of tissue expanders.
For oncological defects, clear margins of malignancy are verified, before reconstruction, especially in repairs involving large areas of undermining, as these may be then need to be removed in an oncological re-excision. Local wound care or temporizing reconstruction with dermal regeneration templates, allografts, or xenografts is considered when clear margins cannot be confirmed at the time of tumor excision.
The hair line can be distorted by excessive tissue undermining and recruitment. It should also be addressed when designing rotational flaps or planning scalp tissue expansion. Defects involving the hair line may require reconstruction with hairbearing and non-hair-bearing tissues.
3. The Patient
Before considering complex procedures or multi-stage scalp reconstruction, it is paramount to assess the patient’s overall health, level of function, compliance, and personal preference. Patients with significant comorbidities may not be candidates for prolonged surgical procedures. Patients’ compliance and ability to keep pressure off the surgical site are required after pressure ulcer reconstruction. Individuals undergoing tissue expansion need to cope with a lengthy process, some level of pain, and significant disfiguration. Some may prefer a singlestage surgical treatment instead.
Oncological patients require special considerations. Attention is given to preoperative chemotherapy agents that could compromise wound healing. Nutritional state is checked. Patients may present for reconstruction before radiation and/or chemotherapy and require a reliable shortterm reconstructive approach that will allow them to continue cancer treatment in a timely fashion. Therefore, tissue expansion is usually not an initial option. In severe cases, patient’s life expectancy may influence the choice of reconstruction.
Reconstructive Options
Primary Closure.
Primary closure is possible for scalp defects up to 3 cm in diameter. The scalp has a rich vascular supply, which allows for some degree of tension. On the other hand, the galea is relatively inelastic and prevents tissue recruitment. Undermining the surrounding tissues at the subgaleal plane yields some mobilization, which may be sufficient for wound coaptation. Open wounds are irrigated and devitalized or fibrotic edges are sharply debrided before closure.
Secondary Closure.
Although this is an option for all soft tissue wounds, wound healing by secondary intention should be discouraged in the scalp, unless other reconstructive techniques are not available to the surgeon. Complete wound healing can be a lengthy process, depending on its size and depth. Exposed calvarial bone devoid of pericranium is prone to osteomyelitis and stable spontaneous coverage is unlikely. Finally, the hairless scar that results is cosmetically problematic.
Skin Grafts.
Skin allografts and xenografts can be used for temporary closure of scalp defects of any size. One to two weeks may pass before the graft is lost by rejection. During this time, for instance, conclusive pathological information can be obtained on the resected specimen (benign versus malignant tumor, margin status, need for radiation therapy, etc.) before a final reconstructive plan is implemented. In other occasions, the surgeon may not be certain of the ability of a scalp wound to accept a skin graft and heal, most commonly because of lingering infection, injury from radiation therapy, tissue ischemia, or malnutrition. In such cases, a trial period with xeno- or allografts can provide the wound with time to improve and at the same time be informative to the surgeon, with no expense of the patients’ donor sites. Immediate or early loss of the xeno/allograft indicates that the wound bed is not healthy, while good take of the xeno/allograft prompts the surgeon to proceed with the definitive reconstruction.
Split-thickness autografts are used in scalp reconstruction for coverage of the primary defect, the secondary defect (donor area of a local flap), or a flap devoid of skin (pericranial, muscular, omental, etc.) and require a healthy vascularized bed for success. Alopecia and some degree of color mismatch are expected. When placed over the calvarium, an intact pericranium is preferred (Figure 31.4). Skin grafts over pericranium, however, have low tolerance to shearing forces and are prone to wound breakdown. Burring down the outer cortical layer of the skull to induce the formation of granulation tissue prior to grafting is also discouraged for the same reason. When available, pericranial or temporoparietal fascial flaps can be used to recreate a thicker vascularized wound bed and decrease the wound’s depth. For best cosmetic results, skin autografts to the scalp are not meshed.
Dermal Regeneration Templates.
Integra (Integra LifeSciences, Plainsboro, NJ) is a synthetic bilaminate composed of a collagen lattice covered with a thin silastic sheet, which renders it impermeable to water. Vascularization of the deeper layer usually occurs in a few weeks, at which point the silastic sheet is removed and a thin split-thickness skin autograft is applied. Advantages of the use of Integra in scalp reconstruction include the simplicity of initial wound management, the potential as a wound-temporizing technique, the increase in thickness of the wound bed allowing for more stable coverage with a subsequent autograft, and the decrease in donor site morbidity because a thinner skin graft is needed. Disadvantages may include the availability and cost of the product, the relatively long period required with a pressure dressing, and alopecia.
Local Flaps.
Local scalp flaps can be designed as partial or full thickness. Partial-thickness flaps, such as pericranial and galeal flaps, are most useful to create a vascularized wound bed when full-thickness scalp flaps (FTSFs) are not available or desired. Unlike coverage with dermal regeneration templates, which require time for vascular ingrowth, partial-thickness scalp flaps can be covered with a skin graft immediately. The pericranial flap includes the periosteum of the skull along with the overlying loose areolar tissue (Figure 31.5). The flap should be based on one of the named scalp vessels or originate from their general vicinity. Perfusion across the midline, often meager, can be enhanced by including the galea with the flap. This galeal-pericranial flap is a much more robust parcel of tissue that can be dissected off the neighboring scalp, placed over the bare skull, and covered with a skin graft.3,4 This dissection is tedious and bloody and may result in some alopecia at the donor site.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree