Introduction526
INTRODUCTION
REACTIONS TO TRAUMA AND IRRITATION
DERMATITIS ARTEFACTA
Histopathology
DECUBITUS ULCER
Histopathology47
FRICTION BLISTERS
Histopathology52
Electron microscopy59
CALCANEAL PETECHIAE (‘BLACK HEEL’)
Histopathology62. and 75.
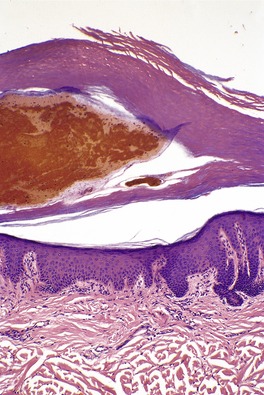
Fig. 21.1
REACTIONS TO RADIATION
RADIATION DERMATITIS
Early radiation dermatitis
Subacute radiation dermatitis
Eosinophilic, polymorphic, and pruritic eruption of radiotherapy (EPPER)
Late radiation changes
Radiation recall dermatitis
Histopathology86
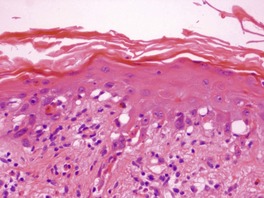
Fig. 21.2
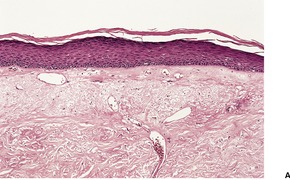
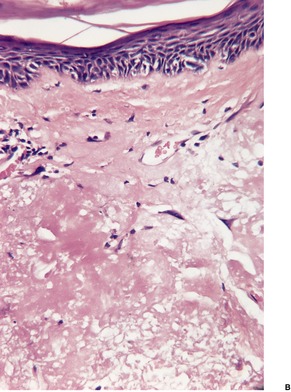
Fig. 21.3
REACTIONS TO HEAT AND COLD
THERMAL BURNS
Histopathology139
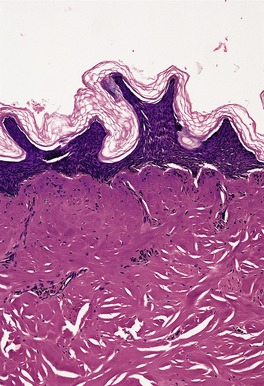
Fig. 21.4
ELECTRICAL BURNS
Histopathology
FROSTBITE
Histopathology
CRYOTHERAPY EFFECTS
Histopathology149
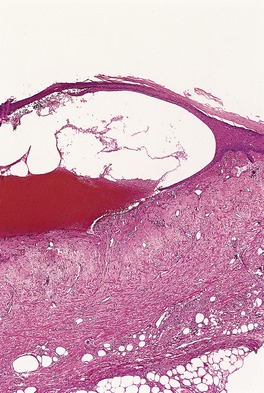
Fig. 21.5
POLYMORPHOUS COLD ERUPTION
Histopathology152
REACTIONS TO LIGHT (PHOTODERMATOSES)
PHOTOSENSITIVE GENODERMATOSES
Xeroderma pigmentosum
Cockayne’s syndrome
Bloom’s syndrome
Hartnup disease
Rothmund–Thomson syndrome
Smith–Lemli–Opitz syndrome
Kindler’s syndrome
PHOTOTOXIC DERMATITIS
Note: A full list of drugs is included in Litt JZ. Drug Eruption Reference Manual, 14th edn. London: Informa Healthcare, 2008.
Phytophotodermatitis
Contact with various plants, including vegetables and topical herbal therapies
Pseudoporphyria pattern
Acitretin
Hemodialysis
Amiodarone
Isotretinoin
Ampicillin-sulbactam
Ketoprofen
Aspirin
Mefenamic acid
β-Lactams
Nabumetone
Bumetanide
Nalidixic acid
Cefepime (?)
Naproxen
Celecoxib
Oxaprozin
Chlorthalidone
Pravastatin
Ciprofloxacin
Pyridoxine
Cola consumption
Rofecoxib
Contraceptive pill
Sulfonamides
Cyclosporine (ciclosporin)
Tanning beds
Dapsone
Tetracyclines
Etretinate
Torsemide
5-Fluorouracil
UVB phototherapy
Flutamide
Vinblastine
Furosemide
Voriconazole
Photo-onycholysis
Captopril
Sparfloxacin
Indapamide
Thiazides
Other drugs
Amiodarone
Retinoids
Atorvastatin
Sitafloxacin
Carbamazepine
Sparfloxacin
Doxycycline
Sulfonamides
Enoxacin
Tetrazepam
Fleroxacin
Thiazides
NSAIDs
Thioxanthenes
Ofloxacin
Voriconazole
Phenothiazides
Topical agents
Coal tar and derivatives
Perfumes
Psoralens
Sun barrier preparations
Textile dyes
Aromatherapy (bergamot)
Histopathology
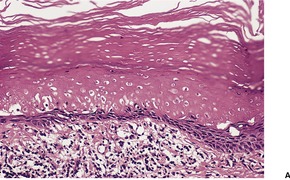
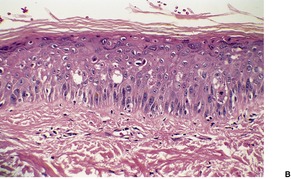
Fig. 21.6
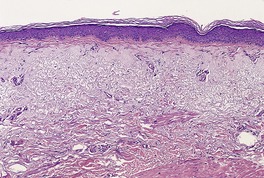
Fig. 21.7
Electron microscopy
PHOTODYNAMIC THERAPY
PHOTOALLERGIC DERMATITIS
NSAIDs
Ibuprofen
Furosemide
Diuretics
Hydrochlorothiazide
Hydrochlorothiazide combined with ACE inhibitor
Ca2+ channel blockers
Amlodipine
Nifedipine
Diltiazem
Antiarrhythmic
Amiodarone
Antibiotic-associated photodrug reactions to doxycycline and the quinolones are well known and rarely biopsied.
Topical agents
Fragrances
Benzocaine
Coumarin derivatives
Piroxicam gel
Sunscreens
Ketoprofen
Benzophenones in other products
Compositae
Systemically administered drugs
Alprazolam
Ketoprofen
Amlodipine
Lomefloxacin
Ampiroxicam
Nifedipine
Azathioprine
Piketoprofen
Capecitabine
Piroxicam
Celecoxib
Pyridoxine hydrochloride (vitamin B6)
Chlordiazepoxide
Quinapril
Chloroquine
Quinidine
Chlorpromazine
Quinine
Cyclamates
Ranitidine
Demeclocycline
Rofecoxib
Diphenhydramine
Sertraline (Zoloft)
Droxicam
Sulfonamides
Enalapril
Tegafur
Fibric acid derivatives (such as fenofibrate and clofibrate)
Tetracycline derivatives
Flutamide
Thiazides
Griseofulvin
Tolbutamide
Herbal treatments
Triflusal
Ibuprofen
Voriconazole
Itraconazole
Lichenoid photoallergic reaction
Chloroquine
Quinidine
Demeclocycline
Quinine
Enalapril
Thiazides
Flagellate dermatitis
Shiitake mushrooms
Photo-pustular eruption
Antidepressant therapy
Histopathology187. and 221.
HYDROA VACCINIFORME
Histopathology
POLYMORPHIC LIGHT ERUPTION
Histopathology331.341. and 352.
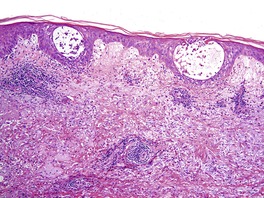
Fig. 21.8
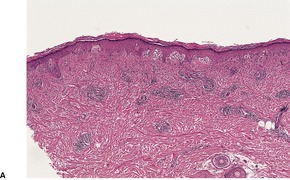
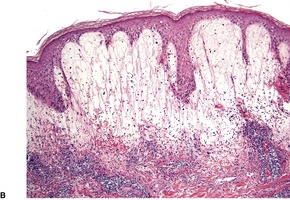
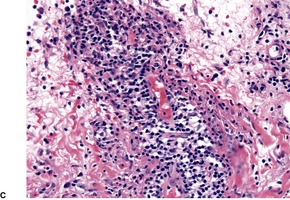
Fig. 21.9
ACTINIC PRURIGO
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Reactions to physical agents
Various physical agents such as trauma, heat and cold, radiation, and light may cause lesions in the skin. 1 This is not an exhaustive account of all the reactions that can theoretically result from physical injuries, as some are of little dermatopathological importance. Several entities produced by physical agents have been discussed in other chapters because they possess a distinctive histopathological pattern. For completeness, brief mention is made of these entities in the introductory discussion to the relevant physical agent.
There are many cutaneous lesions which result from trauma and irritation in the broadest meaning of the words. 2 However, some of these, such as abrasions, bruises, lacerations and the like,3. and 4. are of no dermatopathological interest; animal and human bites also belong to this category. 5 In contrast, calcaneal petechiae (‘black heel’) and related traumatic hemorrhages in some other sites are important because they are sometimes mistaken clinically for a melanocytic lesion. Dermatitis artefacta is also an important entity because its clinical recognition is often delayed. Many quite diverse lesions can result, particularly when foreign materials are injected into the skin.
Entities discussed in other sections include scars (see p. 318), corns and calluses (see p. 701), and acanthoma fissuratum, a reaction to chronic friction from ill-fitting spectacles or other prostheses. Granuloma fissuratum presents with irregular acanthosis bordering on pseudoepitheliomatous hyperplasia and is accordingly discussed with that tissue reaction pattern on page 670.
Traumatic lesions are now being seen from a diverse group of agents. They include airbags in cars,6. and 7. cupping, resulting from a localized vacuum applied against the skin and used in Asia as an alternative therapy for a variety of ailments, 8 and torture. 9 Prayer marks are sometimes seen on the foreheads and knees in Muslims who pray for prolonged periods. 10 Skin thickening, lichenification, and hyperpigmentation are the important consequences. 10
Dermatitis artefacta is a self-inflicted dermatosis occurring in malingerers, the mentally handicapped, and in association with various psychiatric disturbances, particularly personality disorders.11. and 12. The definition is sometimes extended to include factitious lesions resulting from child abuse.13. and 14. Two reviews of the cutaneous manifestations of child abuse have recently been published.15. and 16. Abuse by burning comprises approximately 6–20% of all child abuse cases.16. and 17. Ecchymoses and bruising are further signs suspicious for abuse. 16
Dermatitis artefacta may take many different forms, depending on the injurious agent used.11.18.19.20. and 21. Crusted erosions, ulcers, keloids, scars, psoriasis-like lesions, chemical and thermal burns, hair cutting and shaving are among the many lesions encountered.22.23.24.25.26.27.28.29. and 30. Rarely it may masquerade as pyoderma gangrenosum. 31 In younger persons more than 50% of the lesions are on the head and neck.24. and 26. Bullous lesions on the lower legs were reported in an adolescent, but the mechanism of their production was never elucidated. 32 The distribution and shape of these various lesions may be bizarre. Usually there are lesions in different stages of evolution. Deep abscesses, cellulitis, lipogranulomas, and other granulomatous lesions may result from the injection of various substances. 33
It has been proposed that some ulcerations in patients with reflex sympathetic dystrophy may be of factitial origin. 34
The terms dermatitis neglecta, ‘terra firma-forme’ dermatosis, and keratoderma simplex35 have been used for acquired, asymptomatic plaques, often on the neck, resembling a pigmented lesion, a verrucous nevus, or acanthosis nigricans. They rub off with alcohol (see p. 299). 36
A diagnosis of dermatitis artefacta is not often made in the absence of supporting evidence from the clinician. The reasons for this include the lack of histopathological specificity in most instances, the medicolegal implications of making such a diagnosis, and the traditional fear among doctors of missing an organic disease. 37
There is little limit to the type of lesion that may be found: they may take the form of abrasions, excoriations, ulcers, and burns. There may be abscesses, cellulitis, suppurative panniculitis, irritant and allergic contact dermatitis, alopecia (trichotillomania), or hemorrhage. In the presence of granulomas or abscesses a search should always be made for foreign material, including an examination for doubly refractile particles. Electron-probe microanalysis may assist in the identification of foreign material found in tissue sections. 38
Telangiectasia of vessels in the dermis has been reported as a response to persistent local trauma, although in the cases reported the trauma was not intentional but resulted from recreational pursuits. 39
Decubitus ulcers (bedsores) are a worldwide healthcare problem affecting tens of thousands of patients. 40 They usually develop over pressure areas such as the sacrum, the greater trochanter, and the heels, usually in individuals confined to bed for long periods.41.42.43. and 44. Prolonged pressure is thought to compromise the vascular supply of the affected areas, although the pathogenic steps leading to ulceration have not been investigated in detail.45. and 46. Several clinical stages have been identified. There is a preceding erythematous stage (blanching and non-blanching) and a late stage in which a black eschar forms. 47
Uncommonly, a cutaneous metastasis may mimic a decubitus ulcer. 48
Pressure ulcers are difficult to treat, so prevention is an important strategy. The main treatment principles include reduction of pressure, friction and shear forces, local wound care, surgical debridement of necrotic tissue, and management of any secondary infection. 49 A recent randomized trial found that traditional resin salve is significantly more effective in the treatment of severe pressure ulcers than cellulose polymer gauzes. 49
The earliest changes are in the upper dermis where the vessels become dilated and the endothelial cells swollen. A perivascular round cell infiltrate forms in the papillary dermis together with vascular engorgement, formation of platelet aggregates, and perivascular hemorrhage. Mast cells are increased in number. 50 The epidermis and appendages become necrotic. A subepidermal bulla forms prior to epidermal necrosis, if the process is acute. There is abundant fibrin. 51 In some cases of low-grade, chronic pressure, epidermal atrophy will result. Full-thickness dermal necrosis with the formation of an eschar may develop where the skin is thin and bony prominences are close to the surface. 47 Re-epithelialization is always very slow when the epidermis and appendages have both been destroyed and there is endarteritis of small arteries; grafting is usually required.
Atypical decubital fibroplasia is a pseudosarcomatous proliferation of cells with fibrinoid necrosis, reactive fibrosis, and fat necrosis (see p. 818).
Friction blisters are produced at sites where the epidermis is thick and firmly attached to the underlying dermis, such as the palms, soles, heels, and back of the fingers.52. and 53. A common site is the heel, where blisters are caused by ill-fitting footwear. 54 Friction blisters are an uncommon manifestation of dermatitis artefacta. 52 If the trauma is prolonged and severe or the skin is thin, erosions will occur. Recovery is rapid and complete within a few days. 55
There is an intraepidermal blister due to a wide cleft which is usually just beneath the stratum granulosum. The roof of the blister consists of stratum corneum and stratum granulosum and a thin layer of amorphous cellular debris. 56 The keratinocytes in the base of the blister show variable edema, pallor, and even degenerative changes. 57 There is only a sparse perivascular inflammatory cell infiltrate in the papillary dermis. Friction blisters differ from suction blisters which are subepidermal in position. 58
Mitotic activity commences in the base within 30 hours and there is rapid regeneration with the return of a granular layer in 48 hours. 55
The earliest change is intracellular edema, most noticeable at the cell periphery. The membranes of some cells rupture, allowing escape of some of their contents into the extracellular space.
‘Black heel’, which is usually bilateral and roughly symmetrical, consists of a painless, petechial eruption on the heels.54.60.61. and 62. There is speckled, brownish-black pigmentation that may be mistaken for a plantar wart or even a melanoma. 63 It should not be forgotten that melanoma can occur on the heel. 64
Calcaneal petechiae appear to be traumatic in origin. Their formation probably follows a pinching force imparted by shoes at the time of sudden stopping, such as occurs in the course of basketball, tennis, and other sports.63. and 65.
Lesions comparable in appearance, pathogenesis, and pathology occur in other situations.66. and 67. They include jogger’s toenail, 68 weeder’s thumb, 69 and playstation thumb. 70 They may follow the wearing of new shoes, or the pricking of a blister with a needle. 71 The term post-traumatic punctate hemorrhage has been proposed as a unifying term. 66 Subungual blood may also be the presenting sign of an underlying tumor of the nail bed. 72 There are many causes of splinter hemorrhages of the nails. Trauma is the most common cause, but systemic illnesses, including cardiovascular, renal, and pulmonary diseases, are other etiological agents. Idiopathic cases also occur. 73
Subungual hemorrhage produced by acitretin has also been reported. 74
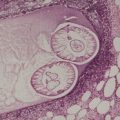
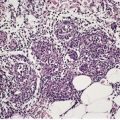
The pigmentation in calcaneal petechiae results from lakes of hemorrhage in the stratum corneum (Fig. 21.1). The red cells are extravasated into the lower epidermis from dilated vessels in the papillary dermis. They undergo transepidermal elimination during the progressive maturation of the epidermis and overlying stratum corneum.
Traumatic hemorrhage involving the stratum corneum. The lesion was removed because of a clinical suspicion that this was a melanoma. (H & E)
Red cells may also be found in the stratum corneum following trauma to the palms, soles, and subungual region. Hemorrhage also occurs into the parakeratotic layer overlying the digitate papillomatous projections in warts. The hemoglobin in these deposits can be demonstrated with either the benzedine stain or the patent blue V stain. 76
The early (acute) effects of X-irradiation differ markedly from those that develop many months or years later. In the skin, the terms ‘acute radiodermatitis’ and ‘chronic radiodermatitis’ have traditionally been used for these respective stages. A subacute form has also been described with features resembling those of acute graft-versus-host disease. 77 All three stages are discussed below under the general heading of radiation dermatitis.
The effects of ultraviolet radiation are quite different. Ultraviolet-B radiation (UVB) produces apoptosis of keratinocytes (‘sunburn cells’), spongiosis, and eventual parakeratosis. Endothelial cells in the superficial vascular plexus enlarge and there is some perivenular edema. 78 Langerhans cells in the epidermis are reduced in number for several days after the exposure. Ultraviolet-A radiation (UVA) produces only mild swelling of keratinocytes and mild spongiosis but no sunburn cells. 78 Exposure to ultraviolet radiation, both UVA and UVB, in commercial tanning salons, has the potential to become a major public health problem in the future. Skin burns, which may predispose to the development of skin cancer later in life, have been reported in a significant number of users. 79
Radiofrequency energy is used in a catheter ablation technique for the treatment of a variety of cardiac arrhythmias. If the procedure is prolonged, a localized acute radiodermatitis may result.80. and 81. A tender or pruritic erythematous plaque develops several days later. Histologically, there are scattered apoptotic keratinocytes resembling a mild phototoxic dermatitis (see below). New collagen production is stimulated by radiofrequency-based devices designed to reduce cutaneous wrinkles. 82 The changes are quite subtle. 82
Nuclear accidents are fortunately quite rare. Neutron and gamma rays may be released in such circumstances. 83 DNA breaks occur in the cells as a consequence of radiation exposure resulting in confluent apoptosis and necrosis of epidermal cells and involvement also of appendageal epithelium. 83
There is a common response to the different types of radiation that affect the skin, although the severity of the changes varies with the total dose, its fractionation, and the depth of penetration of the radiation.84.85. and 86. The use of megavoltage therapy for deep tumors has resulted in some sparing of the skin, although fibrosis in the deep subcutaneous tissues may result.86. and 87.
The advent of coronary angioplasties and stenting has resulted in several cases of radiation dermatitis as a consequence of excessive radiation, usually associated with a prolonged fluoroscopy-guided procedure. Lesions are usually on the upper back.88.89. and 90. Presentations include a vascular lesion, a morphea-like lesion, skin necrosis, or an unexplained ulcer.89. and 91. As already mentioned (see above) radiofrequency energy used in various cardiac ablation techniques can also produce localized radiodermatitis.
There is a well-defined progression of changes following irradiation of the skin. 86 These are usually divided into early changes (acute radiodermatitis) and chronic changes (chronic radiodermatitis) arising many months or years after the initial exposure.92. and 93. An intermediate (subacute) stage is also recognized. The complications of ionizing radiation have recently been reviewed. 94
In the weeks following irradiation there is variable erythema, accompanied by edema in the more severe cases. 86 This is followed by epilation and hyperpigmentation. Severe changes such as vesiculation, erosion, and ulceration are not seen very often in these days of more precisely controlled dosage; they may occur after accidental exposures. 95 The National Cancer Institute in the United States has produced a clinical grading system (1–4) for the manifestations of acute radiation dermatitis. 94
Subacute radiation dermatitis, which occurs weeks to months after radiation exposure, was first described in 1989 by LeBoit. 77 Further localized cases have been described as a consequence of radiation from fluoroscopy during coronary artery procedures.98. and 99. It is a histological imitator of acute cutaneous graft-versus-host disease (see below).77. and 98.
This complication of radiotherapy for cancer, particularly of the breast, has a unique clinicopathological profile. It has been diagnosed in the past as erythema multiforme and bullous pemphigoid following radiotherapy. It has received little attention in the literature, despite the ability of one institution to collect 14 cases. 100 The eruption is polymorphic and pruritic. The eruption usually occurs during radiation, but late onset has been recorded. 101 It is considered further with the spongiotic reaction pattern as this characterizes the histological pattern (see p. 97).
The chronic effects progress slowly and are usually subclinical in the early stages. 86 It seems that at least 1000 rads are required to produce chronic radiodermatitis. 102 The final changes resemble poikiloderma, with atrophy, telangiectasias, hypopigmentation with focal hyperpigmentation, and loss of appendages. 103 Similar changes have been observed in survivors of the Chernobyl accident, when reviewed 15 years later. 104 The affected skin is very susceptible to minor trauma, which may lead to persistent ulceration. 105
A small proportion of patients develop squamous106. and 107. or basal cell carcinoma 15 years or more after irradiation.108. and 109. Rarely, fibrosarcomas have been reported: their diagnosis might not stand up to scrutiny with the immunoperoxidase markers available today. Basal cell carcinomas are more common following irradiation to the head and neck region. 110 Sometimes the tumors that develop are quite aggressive and there is a higher risk of metastasis with any squamous cell carcinoma that develops in the skin following irradiation than with cutaneous squamous cell carcinoma in general. 93 An absorbed dose of at least 2000 rads is required. 111 The risk of cutaneous cancer following superficial grenz therapy is very small indeed.112. and 113.
Radiation recall dermatitis is the development of an inflammatory reaction in a previously irradiated field, precipitated by the administration of certain drugs, usually cytotoxic drugs administered intravenously.114.115. and 116. A similar reaction has been induced by ultraviolet radiation in a patient who had previously received total body irradiation and chemotherapy. 117
The early changes of radiation dermatitis are not commonly seen as there is usually little reason to perform a biopsy. There is some vacuolization of epidermal nuclei and cytoplasm with some degenerate keratinocytes. Inhibition of mitosis occurs in the germinal cells of the epidermis and pilosebaceous follicles. The follicles soon pass into the catagen phase. Later there is hyperpigmentation of the basal layer. The blood vessels in the papillary dermis are dilated and their endothelial cells are swollen. There is edema of the papillary dermis and extravasation of red blood cells and fibrin. Thrombi composed of fibrin and platelets may form in some vessels. Only a small number of inflammatory cells are present and these are usually dispersed and not perivascular in location.
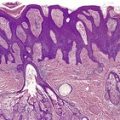
In subacute radiation dermatitis there is a lichenoid tissue reaction (interface dermatitis) with basal vacuolar change and apoptotic keratinocytes (Fig. 21.2). Lymphocytes, which are predominantly CD8+ and express TIA-1, a cytotoxic granule protein in T cells, are found in a superficial perivascular location in the dermis and also in the epidermis in close apposition with apoptotic keratinocytes (‘satellitosis’). There may be a fibrinopurulent crust on the epidermal surface. 98
Subacute radiation dermatitis. There is an interface dermatitis with some basal vacuolar change. (H&E)
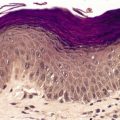
In the late stages the epidermis may be atrophic with loss of the normal rete ridge pattern and the development of focal basal vacuolar change. Sometimes there is overlying hyperkeratosis. Dyskeratotic cells are usually present. The main changes are in the dermis where there is swollen, hyalinized collagen showing irregular eosinophilic staining (Fig. 21.3). This results from marked up-regulation of collagen synthesis. 118 Atypical stellate cells with large nuclei containing clumped chromatin (radiation fibroblasts) are invariably present. 119 Most of these cells express factor XIIIa;120. and 121. a few express CD34. 120 There are telangiectatic vessels in the upper dermis with marked dilatation of their lumen and swelling of the endothelial cells. Vessels are generally reduced in number. Those near the dermal–subcutaneous junction may show varying degrees of myointimal proliferation. Small arterioles and venules often show hyaline change in their walls, with narrowing of the lumen.
Radiation damage. (A) There is loss of dermal appendages. Blood vessels are telangiectatic. (B) The dermal collagen is altered with several ‘radiation fibroblasts’ (dendrocytes). (H & E)
Pilosebaceous structures are absent in late radiation changes and there is some atrophy of eccrine sweat glands. The arrector pili muscles remain, in contrast to the changes following thermal burns, when they are lost. The surviving muscle fibers are often embedded in a pear-shaped mass of collagen, giving the appearance of a bulbous scar.
Less common findings include ulceration, secondary infection and inflammation, and dysplastic epidermal changes resembling an actinic keratosis. Basal or squamous cell carcinomas may supervene. The deep subcutaneous fibrosis that may follow megavoltage therapy overlies atrophic and degenerated skeletal muscle fibers. 87
There are two broad groups of temperature-dependent skin disorders. 122 The first involves the physiological responses that occur in everyone subjected to extremes of temperature. This group includes thermal burns and cold-related disorders such as frostbite. The other group of disorders involves an abnormal response to heat and cold. Abnormal reactions to heat include erythema ab igne (see p. 343), cholinergic urticaria (see p. 204), heat urticaria (see p. 203), and erythermalgia. 122 There are numerous abnormal reactions to cold such as perniosis (see p. 235), livedo reticularis, cold urticaria (see p. 203), sclerema neonatorum (see p. 464), subcutaneous fat necrosis of the newborn (see p. 463), Raynaud’s phenomenon (see p. 304), and cryoglobulinemia (see p. 200). 122
The following reactions to heat and cold will be considered further:
Thermal burns are an important cause of morbidity and mortality. In children, most burns are scalds from hot liquids, while in adults accidents with flammable liquids are more common.123.124.125. and 126. Burns are sometimes a manifestation of child abuse. 127 Acute lesions have traditionally been classified into first-, second-, and third-degree burns according to the extent of the damage. 128 However, some surgeons categorize burns into superficial and deep types. Unfortunately, even this simple classification is difficult to apply on the basis of physical findings alone. 129 A common but neglected phenomenon is the occurrence of blisters weeks to months following a burn, after initial successful healing of partial-thickness wounds. This ‘delayed postburn blister’ may occur for up to 12 months or more after injury. 130 Its mechanism is unknown but it may be due to shearing forces in an area with reduced numbers of anchoring fibrils. 130
Secondary infections, usually due to Gram-negative bacteria, are an important complication in the acute stage. Alopecia may follow a deep burn to the scalp. 131 A late complication, occurring 20–40 years later, is the development of a squamous cell carcinoma.132. and 133. These tumors have a higher risk of metastasis than the usual squamous cell carcinomas of the skin. 134 Basal cell carcinomas and, rarely, malignant fibrous histiocytomas or malignant melanomas may likewise develop many years after the injury.135.136.137. and 138. Dystrophic xanthomatosis is another rare complication (see p. 962).
Biopsy may be of assistance in evaluating the extent of the lesion and the presence of secondary infection, particularly if this is due to a fungus (e.g. Aspergillus or Mucor) or virus. It should be kept in mind that it may be difficult to assess the depth of dermal damage in the first 24 hours as the changes in the collagen are not always fully developed.
In first-degree burns there may be necrosis involving the upper part of the epidermis. Second-degree burns, also known as partial-thickness injury, vary greatly in their extent (Fig. 21.4). In superficial variants there is necrosis of the epidermis with an exudative crust of fibrin, neutrophils, and epithelial cellular debris. Vertical elongation of epidermal keratinocytes usually occurs. Subepidermal blisters may also form. Dermal damage is mild and superficial, and there is only a sparse inflammatory cell infiltrate. In deep second-degree burns many of the pilosebaceous appendages are destroyed, together with much of the dermal collagen. There is fusion of collagen bundles, which show a refractile eosinophilic appearance. Similar changes may occur in vessel walls and there may be thrombosis. Granulation tissue forms at the interface between damaged and viable tissues, resulting in scarring. Epidermal regeneration develops from surviving epithelial components, particularly the eccrine glands, which undergo squamous metaplasia.
Thermal burn. The dermal collagen is ‘homogeneous’. The epidermis is necrotic and there is focal subepidermal clefting. (H & E)
In full-thickness (third-degree) burns the necrosis involves the entire thickness of the skin, including variable amounts of underlying fat. An inflammatory exudate forms at the junction of the viable and non-viable tissue and granulation tissue eventually forms. The necrotic eschar separates after about 3 weeks. As appendages have been destroyed, the only re-epithelialization that can occur is at the margins or by the application of a skin graft.
The scar that follows deep second-degree and third-degree burns is composed of hyalinized collagen. There is usually a decrease in the elastic fibers. Arrector pili muscles are generally lost, in contrast to their preservation in cases of chronic radiation damage, in which they become embedded in scar tissue (see p. 529). 140
The delayed postburn blister that sometimes forms after initial healing is subepidermal in type. 130 There are no immune deposits.
In the United States, the overall incidence of all burns is approximately 4/1000 per year, but only 3% occur as a result of electrical injury. 141 Electrical burns may be of variable severity, depending on the nature of the current, the voltage, and the extent of contact with the skin. They resemble third-degree thermal burns although in severe electrical burns there is deep vascular damage leading to cutaneous infarcts which are slow to heal.128. and 142. Electrical burns in the mouth result in irregularly shaped ulcers covered by slough. 143 Third-degree burns have been incurred as a result of interferential current therapy used to treat pain and reduce edema. 141
Lightning strikes may be associated with full-thickness burns, linear charring, and branching or ferning marks known as Lichtenberg figures.144. and 145.
The use of controlled electrical currents during diathermy and electrodesiccation produces epidermal necrosis resembling thermal burns. A similar picture results from the use of short-pulse, carbon dioxide laser. 146 It seems, based on animal experiments, that the energy required to vaporize the dermis is greater than that needed for the epidermis. 147In electrical burns, elongated cytoplasmic processes extrude from the basal cells into the cavity that forms by dermoepidermal separation. More superficial keratinocytes in the epidermis may also show vertical elongation. There is also homogenization of the collagen in the upper dermis. In severe electrical burns there is infarction of the entire thickness of the dermis and subcutis, with necrosis of vessel walls in the deeper tissue. 142 Hemorrhage often accompanies this vessel damage. 142
The Lichtenberg figures seen following lightning strikes show subcutaneous hemorrhage. 144
Frostbite occurs when tissue freezes. 122 Usually, an acral part such as a finger, toe, ear, or even the nose is involved. The affected part becomes white or bluish white. 122 A blister forms a day or so after rewarming and this is followed some days later by the formation of a hard eschar. 122 Autoamputation of the affected area eventually occurs. The terms ‘chilblain’ and ‘perniosis’ (see p. 235) are used for a specific lesion that may result from exposure to cold temperatures.
Cold injury has resulted from electronic cooling devices attached to the knee after knee surgery in an attempt to decrease postoperative pain and inflammation. 148 The histological changes were similar to those seen in perniosis. 148
The affected area is necrotic and an inflammatory infiltrate is found at the periphery. Granulation tissue eventually forms at the junction between viable and necrotic tissue.
Cryotherapy using carbon dioxide or liquid nitrogen is commonly used in the treatment of cutaneous tumors and premalignant keratoses. As cryotherapy involves rapid cooling it produces numerous intracellular ice crystals and hence more destruction than does the slow cooling of an area. 122
The effects of cryotherapy on normal skin have been elucidated using volunteers and by studying the skin adjacent to lesions which are removed subsequent to cryotherapy. These studies have shown that different cells vary in their susceptibility to cold injury. 122 For example, melanocytes appear to be particularly sensitive to the effects of cold, an explanation for the hypopigmentation that may follow cryotherapy.
The application of liquid nitrogen results in the loss of cellular outline in the epidermis, which appears homogenized. The upper dermis becomes edematous and a subepidermal bulla forms (Fig. 21.5). If the application is more prolonged, homogenization of the upper dermis also occurs. Similar changes occur in any tumor that is so treated.
Cryotherapy blister. The lesion is subepidermal with luminal hemorrhage. (H & E)
New collagen is laid down in the papillary dermis over several weeks and this may be accompanied by blunting of the rete pegs. Sometimes excessive collagen forms, leading to scarring. In solar elastotic skin, new collagen fibers are often intermingled with elastotic fibers. 150 There may be small foci of giant cell elastoclasis, with multinucleate foreign body giant cells phagocytosing a few of the elastotic fibers. 150 Focal acanthosis or pseudoepitheliomatous hyperplasia may develop, often in relation to the transepidermal elimination of damaged collagen and elastic fibers. 150 These changes may lead to a clinical suspicion that the initial lesion has not responded to the cryotherapy.
Another change that follows cryotherapy is localized hypopigmentation, sometimes with a halo of hyperpigmentation. 151 In either case, there may be some melanin lying free, or in macrophages, in the upper dermis.
Polymorphous cold eruption, a rare, autosomal dominant disorder, is characterized by a non-pruritic erythematous eruption developing after generalized exposure to cold air. 152 The lesions are usually localized to the face and extremities. The eruption is often accompanied by fever, malaise, and headache. It differs clinically and histologically from cold urticaria, with which it is often confused.
There is a superficial and deep mixed inflammatory cell infiltrate composed of lymphocytes, neutrophils, and eosinophils. An infiltrate composed predominantly of neutrophils is present around eccrine sweat glands. There is no vasculitis.
The photodermatoses are a heterogeneous group of cutaneous disorders in which light plays a significant pathogenetic role.153. and 154. This group is sometimes expanded by the inclusion of those conditions, both congenital and acquired, which are exacerbated in some way by light but which are not directly produced by it.155.156.157.158.159. and 160. This expanded concept of light-sensitive dermatoses includes the following diseases.
• Metabolic/nutritional dermatoses:
porphyria
disorders of tryptophan metabolism
pellagra
• Light-sensitive dermatoses:
lupus erythematosus
lichen planus variants
rosacea
Hailey–Hailey disease
Darier’s disease
seborrheic dermatitis
atopic dermatitis
erythema multiforme
pityriasis rubra pilaris
disseminated actinic porokeratosis
herpes simplex infections
• Photodermatoses:
phototoxic dermatitis
photodynamic therapy
photoallergic dermatitis
hydroa vacciniforme
polymorphic light eruption
actinic prurigo
solar urticaria
• Chronic photodermatoses:
persistent light reaction
photosensitive eczema
actinic reticuloid
brachioradial pruritus.
Before considering the various photodermatoses, a brief mention will be made of the complications of therapy using psoralens plus ultraviolet-A light (PUVA therapy). This therapy has been an effective treatment for psoriasis and some other dermatoses for many years. Several studies have shown an increased risk of skin cancers in these patients, particularly those who have received high doses. 164 For example, in one study of patients who had received cumulative UVA doses greater than 2000 J/cm2, 19% had developed squamous cell carcinomas and 46% solar keratoses. 165 Interestingly, none of the 13% of patients without PUVA lentigines (see p. 713) had developed keratoses or squamous cell carcinomas. 165 A Swedish study, published in 1999, found an increased incidence of squamous cell carcinoma in patients treated with PUVA, but no increased risk of melanoma, despite concern that melanomas might be a consequence. 166 However, trioxsalen bath PUVA and newer low-dose regimens may lower the risk of skin cancer developing.167.168. and 169. Human papillomavirus was detected in some of the skin tumors that developed in one patient with PUVA-related lesions. 170
The effects of naturally occurring ultraviolet B (UVB) have been studied in southern Chile at the edge of the Antarctic ozone hole. During periods of increased UVB radiation, sunburn was increased and photosensitivity disorders were slightly increased. 171 Alpine skiing is a recreation that leads to increased ultraviolet exposure. 172 Car travel can also result in ultraviolet exposure (UVA) which may be enough to trigger a flare of polymorphic light eruption. 173
In a study of 203 patients presenting with ‘photosensitivity’, the most frequent diagnoses were polymorphic light eruption (26% of cases), chronic actinic dermatitis (17%), photoallergic contact dermatitis (8%), systemic phototoxicity to therapeutic agents (7%), and solar urticaria 4%. 174 Of the remainder, 22% proved not to have photosensitivity, no diagnosis could be made in 12% because of failure of follow-up, and 4% had allergic contact dermatitis. 174 Photodermatoses occur regularly in African Americans, the most common being polymorphic light eruption.175. and 176.
Photodamage has important consequences. Skin cancer and photoaging are the most important of these effects. 177 The skin naturally uses antioxidants to protect itself from photodamage. Antioxidants such as vitamin C, vitamin E, selenium, zinc, silymarin, soy isoflavones, and tea polyphenols may favorably supplement sunscreen protection and provide additional anticarcinogenic protection. 177 However it seems that some individuals are not interested in the consequences of photodamage. It has been suggested that persons who chronically and repetitively expose themselves to ultraviolet light (UVL) to tan may have a novel type of UVL substance-related disorder.178. and 179. Nevertheless, photodermatoses have a significant impact on the quality of life of some patients, particularly those with actinic prurigo and photoaggravated dermatoses. 180
It is important to note that cases of ‘pauci-inflammatory’ photodermatitis occur. 181 Despite conspicuous clinical lesions, the histological changes are mild with telangiectasia and sparse perivascular lymphocytes. 181
The account that follows will be confined to the photodermatoses. Solar urticaria has been discussed with the urticarial reactions (see p. 203). The following entities will be discussed in turn:
• photosensitive genodermatoses:
xeroderma pigmentosum
Cockayne’s syndrome
Bloom’s syndrome
Hartnup disease
Rothmund–Thomson syndrome
Smith–Lemli–Opitz syndrome
Kindler’s syndrome
• phototoxic dermatitis
• photodynamic therapy
• photoallergic dermatitis
• hydroa vacciniforme
• polymorphic light eruption
• actinic prurigo
• chronic photodermatoses:
persistent light reaction
photosensitive eczema
actinic reticuloid
brachioradial pruritus.
The photosensitive genodermatoses are discussed here for completeness. Several of them have been discussed in other chapters. Most are exceedingly rare.
Xeroderma pigmentosum is a rare, autosomal recessive genodermatosis characterized by deficient DNA repair and the early development of mucocutaneous cancers, particularly in sun-exposed areas (see Ch. 9, p. 274). A severe sunburn reaction usually occurs before the age of 2.
Cockayne’s syndrome is characterized by growth retardation, photosensitivity, deafness, mental deficiency, large ears and nose, and sunken eyes. 182 Two types have been identified: Cockayne’s syndrome type 1 (OMIM 216400) due to a gene defect on chromosome 5q12 that encodes the group 8 excision-repair cross-complementing protein (ERCC8); and Cockayne’s syndrome type 2 (OMIM 133540) due to a defect in the ERCC6 gene on chromosome 10q11, involving cross-complementing group 6.
Bloom’s syndrome (OMIM 210900), also known as congenital telangiectatic erythema, is a rare autosomal recessive disorder due to a defect in the RECQL3 gene on chromosome 15q26.1. It is characterized by a telangiectatic, photosensitive facial eruption, stunted growth, proneness to respiratory and gastrointestinal infections, and predisposition to the early development of malignancies. 183 It is discussed with the poikilodermas (see Ch. 3, p. 67).
This syndrome (OMIM 270400) is an autosomal recessive disorder caused by mutations in the DHCR7 gene on chromosome 11q12–q13 which result in a deficiency in the enzyme 7-dehydrocholesterol reductase (07-DHCR) which catalyses the final step in cholesterol biosynthesis. It is characterized by learning disability associated with one or more of the following features: failure to thrive, dysmorphic facies, cataracts, congenital heart disease, hypospadias, second/third toe syndactyly, and severe photosensitivity.162. and 184. The sensitivity is to the UVA spectrum. 163
Kindler’s syndrome (OMIM 173650) is a rare autosomal recessive genodermatosis characterized by trauma-induced blistering, poikiloderma, and varying degrees of photosensitivity. 185 The gene responsible, KIND1, encodes a protein called kindlin-1 which is involved in linkages of other structures to the actin cytoskeleton. It leads to defects in keratinocyte adhesion and increased apoptosis. Photosensitivity has an early onset in some individuals. 185 It has been included in the latest classification of epidermolysis bullosa, as a variant of this condition (see p. 67).
Phototoxicity is the damage induced by ultraviolet and/or visible radiation as a result of contact with or the ingestion of a photosensitizing substance. 186 It does not depend on an allergic reaction and is therefore akin to an irritant contact dermatitis. 187Phytophotodermatitis is a photosensitivity reaction, usually phototoxic in type, which results from contact with plants containing psoralens and other furocoumarins.188.189. and 190. The families Umbelliferae and Rutaceae contain many phototoxic species. 191 They include celery,188.192. and 193. parsnips, carrots, fennel, dill, giant hogweed, zabon (Citrus maxima), 191 limes, and lemons. 194 Herbal therapies applied to the skin can induce a phytocontact dermatitis. 195 Phytophotodermatitis in children is sometimes mistaken for child abuse. 196
Clinically, phototoxic reactions resemble an exaggerated sunburn reaction with dusky erythema; when severe, vesiculation may occur, followed by desquamation and hyperpigmentation.197.198. and 199. Two patterns of reaction are seen: an immediate reaction, which follows the ingestion of the photosensitizing agent and involves exposed areas such as the face, ears, V-area of the neck, and dorsum of the hands; 200 and a delayed reaction, which follows contact with psoralens and peaks after 2 or 3 days. The reaction is limited to the site of contact with the photosensitizing agent. Prominent pigmentation lasting for weeks or months may follow phytophotodermatitis. 188 In some instances, the preceding erythematous phase may go unnoticed. 189 Chronic changes include wrinkling, atrophy, telangiectasia, and the formation of keratoses.
Another presumed phototoxic reaction is the formation of subepidermal bullae, first reported in patients with chronic renal failure receiving high doses of furosemide (frusemide). 201 Other drugs which may produce this pseudoporphyria reaction (see p. 498) include naproxen, 202 oxaprozin, pravastatin, mefenamic acid, nabumetone, flutamide, dapsone, tetracycline, pyridoxine, nalidixic acid, 203 and vinblastine. 204 A list of drugs producing photosensitivity reactions has recently been published. 205 Prolonged sunbed exposure of chronically sun-damaged skin has also been incriminated in producing pseudoporphyria, although whether there is any phototoxic component remains to be seen. 206 Photo-onycholysis is another rare complication of certain drugs including indapamide, thiazides, captopril, and sparfloxacin.207. and 208.
Ingested drugs capable of causing a phototoxic reaction include, in addition to those mentioned above, phenothiazines, thioxanthenes, 209 thiazides,210. and 211. doxycycline, 212 fleroxacin, 213 ofloxacin, 214 sitafloxacin, 215 enoxacin, 215 sparfloxacin, 215 tetrazepam, 216 non-steroidal anti-inflammatory drugs, 217 voriconazole, 218 retinoids, 217 amiodarone, 219 atorvastatin, 220 carbamazepine, and sulfonamides.197. and 221. Azathioprine therapy appears to photosensitize skin to subsequent UVA radiation. 222 Topical agents associated with phototoxicity include coal tar and derivatives, psoralens, 189 and textile dyes, 223 as well as some perfumes and sun barrier preparations. 224 The causes of a phototoxic dermatitis are listed in Table 21.1. A bullous phototoxic reaction has followed the use of aromatherapy oil containing bergamot which contains furocoumarins. 225 Tobacco smoke is phototoxic. 226
A phototoxic reaction requires the absorption of photons of specific wavelengths by the photosensitizing substance. 221 Energy is dissipated as it returns from an excited state to its ground state. Free radicals, peroxides, and other substances are formed which may potentially damage cellular and subcellular membranes.221. and 227. The action spectrum is usually in the UVA range, although with some ingested substances (e.g. thiazides) it is in the shorter UVB wavelengths.197. and 210. The formation of apoptotic keratinocytes (‘sunburn’ cells) appears to require UVB radiation; tumor necrosis factor-α, p53 protein, and other factors are involved in some way.228.229. and 230. There is some evidence that a p53-independent pathway can also be involved. 231 Melanocytes, in contrast, are much more resistant to ultraviolet-induced apoptosis, due to a high level of antiapoptotic proteins such as Bcl-2. This protein is up-regulated by nerve growth factor produced by keratinocytes exposed to UV irradiation. 232 It is interesting to speculate whether this is the mechanism of the melanocytic hyperplasia seen in chronic sun-damaged skin.
Phototoxicity is more common than photoallergy, from which it differs by being dose related and by its subsidence on removal of the photosensitizer or the ultraviolet radiation. Some drugs produce both toxic and allergic reactions; in others, the mechanism has not been elucidated.
If the photosensitizing agent is applied to the skin there is some ballooning of keratinocytes in the upper dermis, with epidermal necrosis in severe reactions187 and scattered apoptotic keratinocytes (‘sunburn cells’) in mild reactions (Fig. 21.6). There is variable spongiosis. A similar picture is seen in biopsies taken from photopatch test sites in phototoxic states. 233 If the chromophore reaches the skin through the vasculature there may be only minor epidermal changes. 234
(A) Severe phototoxic reaction with epidermal necrosis. (B) In this less severe phototoxic reaction that followed contact with a psoralen-containing plant (phytophotodermatitis), there are scattered ‘sunburn cells’ in the epidermis. (H & E)
In both instances, there is a mild or moderate superficial inflammatory cell infiltrate in the dermis. 234 It is composed predominantly of lymphocytes, although a few neutrophils may be present in severe reactions. Eosinophils are sometimes seen. Although it is usually taught that the dermal infiltrate is both superficial and deep in the light-related dermatoses, this is not always so, particularly in acute lesions. Pigment incontinence is sometimes present, 210 while in late lesions of phytophotodermatitis there is also basal hyperpigmentation.
In bullous phototoxic reactions (pseudoporphyria) there is a subepidermal blister with only rare inflammatory cells in the base (see p. 498). A very occasional eosinophil is sometimes present. In cases of long standing, the blisters may heal with scarring and milia formation. 234 In chronic phototoxic states (bullous and non-bullous), basophilic elastotic fibers extend below the mid dermis (Fig. 21.7). There is also PAS-positive, diastase-resistant material in and around small blood vessels in the upper dermis. Fibroblasts and dendrocytes are also increased in photosensitivity reactions of some duration.
Chronic photosensitivity. There are deeply extending basophilic elastotic fibers but no inflammation. (H & E)
Small amounts of IgG and sometimes C3 are found adjacent to vessels and near the basement membrane zone in chronic states. 234
In chronic phototoxicity there is reduplication of the basal lamina in dermal vessels and fine fibrillar deposits in their vicinity. 234
Photodynamic therapy is used, selectively, in the treatment of some cutaneous malignancies. It involves the use of a photosensitizing agent which is applied to, or accumulates in, malignant tissue, followed by the application of a light source that activates the photosensitizer. 235 This results in the release of toxic oxygen radicals that destroy the malignant cells. Recently, partial-thickness burns have been reported following the ingestion of temoporfin, a second-generation photosensitizer. 236 Acute phototoxicity with urticarial features has also been reported from photodynamic therapy using topical 5-aminolevulinic acid. 237
Photoallergy is increased reactivity of the skin to ultraviolet and visible radiation and is brought about by a chemical agent on an immunological basis.186. and 234. The photosensitizing chemical is usually applied topically to the skin; uncommonly, photoallergic reactions may follow ingestion of a drug.186. and 238.
The usual photoallergic reaction develops 24–48 hours after sun exposure and is a pruritic, eczematous eruption. 239 Lichenification may occur in cases of long standing. Lichenoid papules have been reported in some thiazide-induced photoallergic reactions. 239 Photoallergic dermatitis occurs essentially on light-exposed areas, although there is a tendency for lesions to extend beyond the exposed areas. Regression usually occurs after 10–14 days, although in some cases the condition may persist for long periods and the patients become persistent light reactors (see below). Topical agents that have been implicated in photoallergic reactions include fragrances such as musk ambrette240 and coumarin derivatives, 241 and sunscreens containing benzophenones,242.243.244.245. and 246. oxybenzone, butyl methoxy dibenzoylmethane, 246 methoxycinnamate, 247 or para-aminobenzoic acid and its esters.198.224. and 248. Benzophenones contained in products other than sunscreens can also produce photoallergic reactions. 249 Benzocaine, 250 piroxicam gel, 251 ketoprofen, 252 plants in the Compositae family, and, rarely, psoralens have been recorded as producing photoallergic reactions. The halogenated salicylanilides, which resulted in numerous cases of photoallergic dermatitis over two decades ago, have been withdrawn as topical antibacterial agents. 253 Systemically administered drugs which rarely produce photoallergy include quinine,254. and 255. quinidine,256. and 257. griseofulvin, possibly itraconazole,258. and 259. ranitidine,260. and 261. lomefloxacin, 262 chlorpromazine, 263 piroxicam,264.265. and 266. ampiroxicam, 267 droxicam, 268 piketoprofen, 269 ketoprofen,270. and 271. voriconazole,218. and 272. pyridoxine hydrochloride (vitamin B6),273. and 274. tegafur, 275 flutamide,276. and 277. alprazolam, 278 sertraline (Zoloft), quinapril, 279 triflusal, 280 nifedipine, 281 amlodipine,281. and 282. thiazides, sulfonamides, 283 capecitabine, 284 celecoxib, 285 certain tetracycline derivatives, diphenhydramine, 286 tolbutamide, ibuprofen, 287 herbal treatments, 288 fibric acid derivatives (such as fenofibrate and clofibrate),289.290. and 291. chlordiazepoxide, and cyclamates.224. and 234. The author’s experience of the more common drugs producing photosensitive reactions over the last 5 years is shown in Table 21.2. These observations are based on the consequences of drug cessation but not subsequent rechallenge. Drugs which may produce a lichenoid photoallergic reaction include, in addition to thiazide diuretics (see above), demeclocycline, enalapril, quinine, quinidine, and chloroquine. 217 A flagellate dermatitis, resembling that produced by bleomycin (see p. 394), has been reported after the ingestion of raw shiitake mushrooms.292. and 293. A photo-distributed pustular drug eruption, with overlap features between Sweet’s syndrome and acute generalized exanthematous pustulosis, has been associated with antidepressant therapy. 294 The various drugs producing photoallergic reactions are listed in Table 21.3.
Three factors are required in a photoallergic reaction: a photosensitizing agent, light (usually in the UVA range), and a delayed hypersensitivity response. 186 The absorption of light energy appears to alter the photosensitizing chemical in some way to produce a hapten which attaches to a protein carrier and eventually stimulates immunocompetent cells, producing a hypersensitivity response. 221 Photopatch testing is used to elucidate the offending agent. 295 The standard set of photoallergens has to be updated periodically to accommodate changing formulary of topical preparations. 296
The changes resemble those seen in contact allergic dermatitis and include epidermal spongiosis, spotty parakeratosis, and some acanthosis.200. and 254. Spongiotic vesiculation occurs in severe cases. There is a moderately heavy infiltrate of lymphocytes, mainly in a perivascular location in the upper dermis. A few eosinophils are usually present. There is some exocytosis of these inflammatory cells. In some instances, the dermal reaction differs from that seen in contact allergic dermatitis by deeper extension of the infiltrate. This is particularly so in lesions of long standing. Stellate cells and telangiectasia may be prominent, reflecting the photosensitive component. Conspicuous solar elastotic changes may also develop in chronic cases.
The lichenoid papules show a lichenoid tissue reaction with a superficial and mid-dermal inflammatory cell infiltrate. Spongiosis is sometimes present as well. 217 Changes of photosensitivity (telangiectasia, stellate cells, deep extension of the infiltrate, and increased solar elastosis) are usually present, but they take 3–4 weeks or more to develop.
Hydroa vacciniforme is a rare, debilitating photodermatosis of unknown pathogenesis. 297 It is manifested clinically by the development of erythema and vesicles, on uncovered skin, within 1–2 days of sun exposure.297. and 298. The vesicles heal leaving varioliform scars. Ear mutilation has been reported as a consequence of recurrent lesions with scarring.299. and 300. Oral lesions have been reported.301. and 302.Rarely, there are crusted, non-vesicular lesions. 303 The disease usually begins in childhood and runs a chronic course before remitting in adolescence. 304 Late onset305 and familial cases are exceedingly rare.306. and 307. Coexistence with a malignant lymphoma has been documented. 308 Porphyrins are normal. Lesions may be reproduced in many instances by repeated exposures to UVA radiation.309.310.311.312. and 313. Dietary fish oil, which provides some systemic photoprotection, has produced variable clinical responses in this disease. 314
About 10 years ago, attention was drawn to a hydroa vacciniforme-like eruption associated with latent Epstein–Barr virus (EBV) infection, which had a high propensity to develop cutaneous lymphoma, usually of subcutaneous type.315.316.317. and 318. Latent EBV infection has now been detected in the dermal infiltrate of children with typical manifestations of hydroa vacciniforme.315. and 319. Often, cases associated with EBV have lesions on both sun-exposed and protected areas of the body but there are several reports of EBV-associated hydroa vacciniforme limited to the face. 320 Severe cases are often associated with NK-cell lymphocytosis, hypersensitivity to mosquito bites, and fatal hemophagocytic syndrome. Progression to NK/T-cell lymphoma is another cause of death in these severe cases. 321 It has been suggested that both the typical and atypical forms are part of a spectrum, in which the atypical lesions have the potential to lead to an EBV-associated lymphoid malignancy.315. and 322. Lesions of hydroa vacciniforme can be induced in patients with latent EBV infection by repeated UVA irradiation. 323
The exact status of hydroa estivale is uncertain.324. and 325. It has been regarded in the past as a mild form of hydroa vacciniforme or a childhood form of polymorphic light eruption. 309 Some of the earlier reported cases may have been erythropoietic protoporphyria.326. and 327.
The established lesions of hydroa vacciniforme show intraepidermal vesiculation with reticular degeneration and, later, confluent epidermal necrosis. 309 The vesicles are filled with serum, fibrin, and inflammatory cells. Ulcerated lesions may be associated with superficial dermal necrosis. 326 Vascular thrombosis is sometimes present in these circumstances. Lymphocytes and neutrophils are present at the lower border of any necrotic zone. In addition, there is a superficial and deep perivascular infiltrate of lymphocytes and occasionally a few eosinophils. There are no PAS-positive deposits. 326 A dense peri-eccrine infiltrate of small and medium-sized lymphocytes may be present. 328 In some severe cases a lobular or septal panniculitis may also be present. Healed lesions show variable scarring in the upper dermis. 303
Direct immunofluorescence sometimes shows scattered granular deposits of C3 at the dermoepidermal junction. 309 EBV-encoded RNA (EBER) can be identified in dermal lymphocytes. 328 It was found in 28 of 29 patients in a recent study of patients with hydroa vacciniforme or severe hydroa vacciniforme-like eruptions. 321
Polymorphic light eruption (polymorphous light eruption) is an idiopathic photodermatosis in which lesions of varied morphology appear several hours or even days after exposure to the sun and subside in a further 7–10 days if further exposure is avoided.329.330.331. and 332. Certain populations appear to be genetically predisposed: in Finland, cases with an autosomal dominant mode of inheritance, with reduced gene penetrance, have been reported. 333 Twin studies and genetic modeling have also established a clear genetic influence.334.335. and 336. Polymorphic light eruption affects 10–20% of those living in temperate climates;331.337. and 338. it is much less common in populations living nearer to the equator. 339 The prevalence in the northern hemisphere is about 15%. It is very uncommon in subtropical Brisbane, Australia (author’s personal experience), yet it is not uncommon 1000 miles to the south. 340
The lesions may take the form of small pruritic papules, papulovesicles, or urticarial plaques. 341 The papulovesicular form (pinpoint papular variant) has been regarded as a discrete subset.342.343. and 344. Rarely, lesions may resemble those seen in erythema multiforme or insect bites. 341 Eczematous lesions and lesions resembling those of prurigo nodularis345 have been described,346. and 347. although these variants are not universally accepted as part of the spectrum of polymorphic light eruption. 341 Lesions are usually monomorphous in the same individual. 348 The severity of the disease is highly variable. 349 Sun-related pruritus may rarely precede the onset of the disease. 350 Contact and photocontact allergies are present, rarely, in patients with polymorphic light eruption. 351
Lesions have a predilection for the dorsum of the hands and forearms, the upper part of the arms, the neck, and the face. 352 The face is usually spared in the papulovesicular form. The photodermatosis known as ‘juvenile spring eruption of the ears’, a condition found predominantly in young boys, is a probable variant of polymorphic light eruption. 353‘Lambing ears’, a blistering and crusting disorder of the pinnae of farmers at lambing time, is a variant of juvenile spring eruption with unusual demographics. 354 The mean age of onset of polymorphic light eruption is in the third decade of life. 355 In most series there has been a female preponderance.341. and 356.
Various associations have been reported, some of which may be fortuitous. They include common variable hypogammaglobulinemia, 357 thyroid disease, 358 and lupus erythematosus.359.360. and 361. A recent study of patients with elevated titers of antinuclear antibodies with polymorphic light eruption found no progression to lupus erythematosus in any of the patients after a median follow-up period of 8 years. 362 Lesions have rarely been limited to areas of vitiligo363 or nevoid telangiectasia. 364 Psychological distress has been reported in more than 40% of individuals with the disease. 365
The condition is chronic in nature, although in about half the cases there is diminished sensitivity to sunlight over time, with periods of total remission; light sensitivity increases in some patients.355.366.367. and 368. The phenomenon of eventual tolerance to sunlight is known as ‘hardening’. 369 Accordingly, prophylactic phototherapy using PUVA therapy is used in the treatment of the disease. 370 Some studies suggest that a delayed hypersensitivity reaction to sunlight-modified skin antigens is involved in the pathogenesis.371.372. and 373. Put in another way, polymorphic light eruption is an autoimmune disease against an ultraviolet radiation-induced cutaneous antigen. 374 In normal subjects, ultraviolet exposure leads to a disappearance of Langerhans cells from healthy skin 48–72 hours later and an accumulation of CD11b+ (Mac1) macrophage-like cells, which reportedly produce interleukin-10. In patients with polymorphic light eruption, Langerhans cells do not disappear after ultraviolet exposure and CD11b+ cells, already present at the time of exposure, further increase and invade the epidermis. 375 There is a reduced expression of TNF-α, IL-4, and IL-10 in the UVB-irradiated skin of patients with polymorphic light eruption which is largely attributable to a lack of neutrophils, and is indicative of reduced Langerhans cell migration. 376 In short, there is a defect of UV-induced immunosuppression in this disease. 377 There is normalization of UVB-induced trafficking of Langerhans cells and neutrophils after artificial UVB ‘hardening’. 378 The action spectrum appears to be quite broad and may involve both the UVA and UVB range,379.380.381. and 382. although UVA radiation appears to be the more important.339.383. and 384. There is no significant relationship between the ease of provocation of the disease by UVA and/or UVB radiation and clinical disease severity. 385
The management of polymorphic light eruption primarily involves preventative measures such as avoidance of sun exposure and use of sunscreens with high UVA protection. 386 The use of phototherapy in an attempt to produce ‘hardening’ has been mentioned above. Systemic immunosuppression may be required in severe cases. 386
There is marked variability in the histopathological changes in the various clinical subsets of polymorphic light eruption (Fig. 21.8 and Fig. 21.9). However, a fairly constant feature (in keeping with the mnemonic mentioned on p. 22) is the presence of a dermal inflammatory cell infiltrate which is both superficial and deep, although if early lesions are biopsied it may not extend below the mid dermis. While the infiltrate is predominantly perivascular, there is sometimes a heavy interstitial infiltrate of lymphocytes in the upper dermis in those variants characterized by prominent subepidermal edema. The lymphocytic infiltrate is composed of T cells387. and 388. and there is evidence that these cells are CD4-positive in early lesions and predominantly CD8-positive in later lesions. 389 Various interleukins may act as lymphocyte attractants. 390 Eosinophils are sometimes present in the dermal infiltrate, while in the papulovesicular type a few neutrophils have sometimes been recorded. A rare neutrophil-rich variant occurs.
Polymorphic light eruption. Note the superficial and deep perivascular infiltrate in the dermis. There is subepidermal edema and spongiotic vesiculation. (H & E)
Polymorphic light eruption. (A) There is a superficial and deep perivascular infiltrate of lymphocytes. (B) In this case there is massive subepidermal edema and (C) a tight deep perivascular infiltrate of lymphocytes. (H & E)
Edema of the upper dermis is often present and is quite prominent in plaque-like lesions. In the rare erythema multiforme-like lesions this may be so marked that subepidermal bullae form. 341 However, there is no lichenoid (interface) reaction as occurs in erythema multiforme. Extravasation of red cells is sometimes seen in the upper dermis, particularly in the papulovesicular form. 391 Unlike lupus erythematosus, there are no dermal deposits of acid mucopolysaccharides. 392
The epidermal changes are variable depending on the clinical type. The epidermis may be normal or show slight changes such as very mild spongiosis, focal parakeratosis or acanthosis. In the papulovesicular form there is invariably spongiosis leading to spongiotic vesiculation. 391 There is often some basal vacuolation, but no basement membrane thickening. Scattered apoptotic keratinocytes are often present in the papulovesicular variant. This may be accompanied by exocytosis of lymphocytes and erythrocytes. 391 In the papulovesicular form there may also be claw-like extension of epidermal rete ridges at the lateral boundaries of each papule, reminiscent of lichen nitidus. 344
Direct immunofluorescence gives variable results. Focal perivascular and interstitial deposits of fibrin and perivascular C3 or IgM have all been recorded in a few cases. 393 The lupus band test is negative. 394
Actinic prurigo (Hutchinson’s summer prurigo, 395 hereditary polymorphic light eruption)396. and 397. is a chronic photodermatitis occurring predominantly in North American Indians and in Central America, although people of European origin and Asians are not spared.398.399.400.401.402.403. and 404. Actinic prurigo has been regarded as a variant of polymorphic light eruption, 398 although this has been challenged on the basis of its distinct HLA typing. 405 It is possible that the characteristic actinic prurigo phenotype is determined by this HLA type (DRB1*0407), found in 70% of patients.404. and 406. An association with DRB1*14 has been reported in an Inuit population. 407 Both actinic prurigo and polymorphic light eruption may share a common pathophysiological basis.335.408. and 409. Families with members having either disease have been reported.335. and 410. Light testing gives inconsistent results although the majority are sensitive to UVA light.
Actinic prurigo is characterized by onset in childhood, female preponderance, a familial tendency, which is quite high in some communities, and severe pruritus.398.399. and 411. It appears to be inherited as a dominant trait but with incomplete penetrance. 412 It commences as an eczematous eruption on exposed areas, particularly the face, earlobes, and forearms. 396 Covered areas are involved in some patients, but not others. 413 Papular, plaque-like and prurigo-like papulonodules develop. 399 Lichenification and postinflammatory scarring can be marked. 404 Other features that may be present include an exudative and crusted cheilitis of the lower lip, 414 conjunctivitis, 415 and alopecia of the eyebrows. 398 Primary cutaneous B-cell lymphoma has developed in two patients. 416