Radiotherapy: Introduction
|
The first documented use of radiation as a therapeutic treatment was for a cutaneous malignancy, in a patient with squamous cell cancer of the nose, in 1900. Over the next century, radiotherapy was widely used in the treatment of both malignant and benign disorders of the skin, in both adults and children. As the long-term consequences of radiotherapy became evident, particularly the risk of radiation-induced malignancy, its use in the treatment of children and benign diseases declined. Radiotherapy continues to have a small but important role in the management of benign proliferative diseases of the skin, but is more commonly used as a valuable adjunct or alternative to surgery for both premalignant and malignant lesions.
Radiation Modalities
There are several choices for radiation modalities, some commonly available and others only in specialized centers. The selection is made on the basis of the anatomic location and size of the target, tumor biology, the nature of critical surrounding structures, and availability. In particular, with respect to the cutaneous targets, the depth of the lesion plays a large role in determining the optimal therapy.
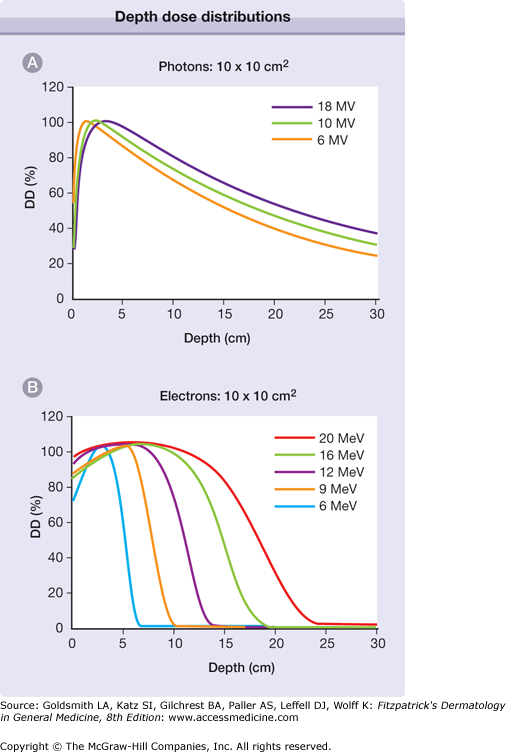
High-energy photons, in the form of γ- or X-rays, are most commonly produced by a linear accelerator (linac) and are available in a spectrum of energies. Incident radiation deposits its energy as it passes through matter, becoming attenuated as a function of distance and the density of the tissue. Higher energy beams deliver increased dose at depth in tissue, and proportionally less at the surface. Lower energy radiation deposits dose primarily at the target surface, sparing deeper matter. The most commonly available treatment energies are in the megavoltage range, which deposit their dose at a range practical for the treatment of targets in human tissue. Such beams were designed to relatively spare surface structures such as skin, which would otherwise be dose limiting, in the interest of delivering higher dose to deeper target structures. This is in contrast to more superficial radiation energies, which are often more appropriate for cutaneous targets. Depth dose curves demonstrating the absorption of X-rays as a function of their energy are demonstrated in Fig. 240-1A. Most linear accelerators in clinical use provide radiation in the range of 6–18 megavolts (MV).1
Figure 240-1
Depth dose distributions. A. The percent of the maximum radiation dose (DD) deposited at depth in tissue (in cm), as a function of the photon (X-ray) energy. As photon energy increases, the percent dose at superficial depth decreases, and the percent dose in deeper tissue increases. B. A similar relationship for electron therapy. In contrast to photons, the percent dose deposited at both superficial and deep tissue increases with electron energy. Note the difference in scale; electron energy is almost completely absorbed at shallower depths, compared to photons.
Orthovoltage X-rays refer to lower energy photons with maximum energy in the range of 125–400 kilovolts (kV), often used in dermatologic applications because the dose at the skin surface dose is maximized. The dose is then rapidly attenuated as the beam penetrates deeper into soft tissue. Half of the incident energy has been absorbed within the first few centimeters, ideal for treating superficial targets and minimizing dose to deeper normal tissue. Orthovoltage X-rays are produced by specialized treatment units, and are not as commonly available as megavoltage treatment units.
Grenz rays are even lower energy X-rays in the range of 5–15 kV, and therefore deposit their dose at more shallow depths than orthovoltage. These were historically used to treat superficial, benign skin disease. In these cases, the majority of the target processes are occurring within 1 mm of the skin surface. Grenz rays are no longer recommended as first-line therapy for routine treatment of benign cutaneous disease.
Charged particle therapy is also commonly used in cancer treatment, including both electrons, which are commonly available, and protons, available at select regional centers. Electrons are the product of the same linear accelerators used to produce megavoltage energy photons. Electron beams are commonly used in dermatologic applications as they are capable of delivering high skin-surface dose, and the deposited dose rapidly falls to negligible values at depth in tissue. Electron-depth dose, like that of photons, is a function of voltage. Higher energy electrons deliver dose to a greater depth, similar to the depth/dose relationship of photon energy. Unlike the skin-sparing properties of higher energy photons, as electron energy increases the skin dose also increases. The depth dose characteristics of electrons are described in Fig. 240-1B.
Protons are charged particles that are the product of large and expensive cyclotrons or synchrotrons, available at a small number of specialized centers. Due to their large mass, there is little side scatter during penetration of a proton beam. The dose is largely delivered within a few millimeters of the end of the particle range (the Bragg peak), rather than at shallower or deeper depths. By modulating the energy of the proton beam, radiation absorption can be more precisely delivered to deep tumor targets, with less incidental radiation of surrounding normal tissue.
Modulation of External Beam Radiation
Radiation treatment planning involves a complex set of decisions regarding the appropriate radiation modality, radiation energy, beam orientation, patient positioning, and the use of treatment devices. The latter can help increase the radiation dose to targeted structures, and block or reduce radiation exposure of normal tissue. “Bolus” is tissue-density material commonly used in radiation treatment of superficial skin malignancies. It can be custom designed in varying thicknesses and applied to the patient’s skin during daily treatment. It serves several potential purposes: for higher energy X-rays (e.g., MV energy), or low-energy electrons, the surface dose is low compared to that in deeper tissue. By placing the appropriate thickness of bolus, the skin dose can be raised to therapeutic levels. This is demonstrated in Fig. 240-2. Another function of bolus can be to attenuate the incident beam to lower the dose to deeper structures, for example, during treatment of a skin cancer on the temple, to decrease the dose that penetrates into the underlying brain. Bolus material can also be used to compensate for complex topography, and smooth the dose distribution for treatment of the skin around the nose and ears, as seen in Fig. 240-3.
Figure 240-2
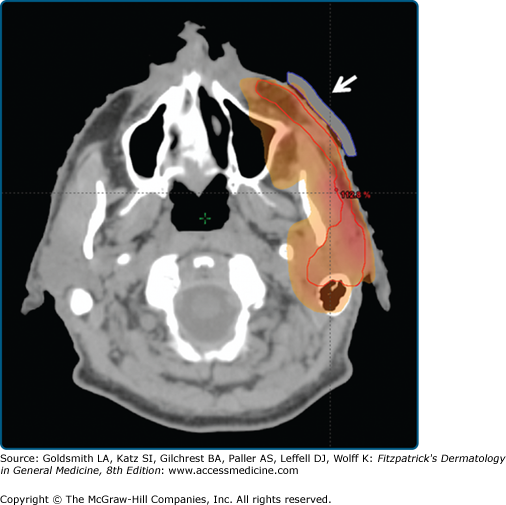
Bolus to increase the skin dose. A 61-year-old male underwent resection of a deeply invasive squamous cell carcinoma overlying the zygoma, with involvement of parotid lymph nodes and facial nerve. The tumor bed was treated, along with the remaining parotid gland and course of the facial nerve, using megavoltage photons. A tissue-density bolus (white arrow) was placed over the tumor bed to increase the skin dose to 100% in that area.
Figure 240-3
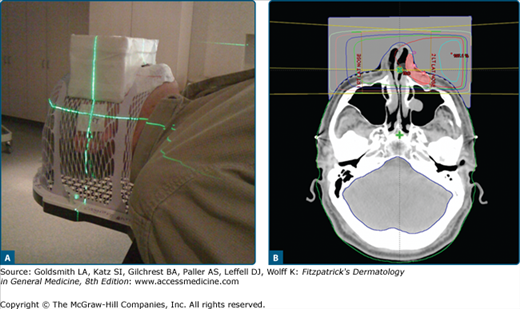
Bolus as a tissue compensator. A deeply invasive basal cell carcinoma was excised from the left nasal ala. A. The patient was immobilized for treatment in a thermoplastic mask. A rectangular, tissue-density, box was constructed overlying the nose to compensate for the irregular tissue contours in this area. B. The patient was treated with right and left lateral megavoltage photon beams, weighted left greater than right. The 100% isodose line (red line) covers the tumor bed (red-shaded area) with full dose at the skin surface and good dose homogeneity.
Beams can be shaped by a variety of devices depending on their energy. Megavoltage treatment beams may be shaped by custom-designed, 7-cm thick, lead alloy blocks to conform to the desired shape; most modern linear accelerators have a multileaf collimator (MLC), which contains sliding leaves of tungsten that conform to the desired treatment aperture. Electron beam radiation is normally blocked with custom-made lead or lead alloy blocks. Orthovoltage radiation is blocked using thinner custom lead shielding, typically placed on the patient’s skin. Examples of blocking devices are shown in Fig. 240-4.
Traditionally, external beam radiation beams are designed using blocks or static MLCs to conform to a target that is delineated clinically or using CT or other imaging. Multiple beams, beam angles, and energies are chosen to avoid specific normal structures, and the dose is calculated with iterative changes until an acceptable plan was generated. An “inverse planned” method is now increasingly used when the tumor target lies in proximity to a dose-limiting structure. Intensity modulated radiation therapy (IMRT) incorporates individual radiation beams that are not static; the aperture changes during the treatment to finely adjust beam fluence. This requires a dynamic MLC, in which the leaves slide during beam-on time. During the pretreatment-planning phase, the treating physician contours the target volume and those of the normal tissue organs at risk. Dose constraints for each contoured structure are chosen, and the physician, physicist, and dosimetry staff use dedicated treatment planning software algorithms to optimize the treatment plan. This is a time intensive, expensive technique that can generate plans to treat complex shaped tumor targets and spare adjacent critical normal tissue structures.
 Brachytherapy
Brachytherapy
Both X-rays and charged particles are considered external beam therapy, or teletherapy, in which radiation is produced external to the patient, and the incident radiation beam shaped and modulated by specialized devices. In contrast, brachytherapy is a commonly used clinical method to deliver radiation dose using close application of radiation sources within the patient. Brachytherapy takes advantage of the inverse square law—the intensity of radioactivity from a point source declines with the square of the distance from that source. If a brachytherapy implant is designed to deliver a specified dose to within 1 cm of the implant, the exposure of surrounding tissue 5 cm distant is approximately 25 times lower.
Interstitial brachytherapy involves the placement of radioactive sources directly within the target tissue. The most common application of interstitial brachytherapy is the implantation of I-125 seeds within the prostate of a patient with prostate cancer. These sources are permanently implanted, and once they have decayed to background activity remain inert. Intracavitary brachytherapy implies that a radioactive source is placed or held within a natural body cavity, typically in a temporary fashion.
Brachytherapy is often classified by the delivered dose rate. Low dose rate (LDR) brachytherapy is a term that implies sources that emit radiation at a rate of up to 2 Gy per hour. This can include temporary implants that remain in place from 10–72 hours or more, as well as permanent implants that deliver radiation dose to the total decay of the implanted sources, often over weeks to months following the procedure. High dose rate (HDR) brachytherapy is terminology used when the dose delivery exceeds 12 Gy per hour. The most common applications of HDR involve the use of remote afterloading techniques. To protect medical personnel from high-activity HDR sources, custom catheters or molds are placed in or on the tumor target, and these are then connected to the afterloading device, which remotely inserts a high activity source, without the necessity of direct handling of the source by personnel. Remote afterloading HDR brachytherapy is normally delivered on an outpatient basis over several treatment appointments. It is advantageous in that there is no exposure to personnel or the public, and source dwell position and duration can be planned to deliver superior dose coverage.
Mechanism of Action
The most common form of radiotherapy used in clinical practice are X-rays, or γ rays. Both represent photon particles or electromagnetic waves that differ only in the method of their generation: γ rays are emitted by nuclear reactions, whereas X-rays are emitted by energy transitions in orbital electrons. Ionizing radiation includes that part of the electromagnetic spectrum of sufficient energy to impart energy to target tissue by the ejection of orbital electrons. This is the primary means of energy absorption in human tissue following exposure to therapeutic radiation. Since cells are largely composed of water, it is in water molecules that the majority of the ionization occurs and the result is the generation of short-lived free radicals, such as hydroxyl radicals. The effectiveness of radiation in tissue is therefore dependent upon the availability of oxygen. This is clinically manifest in reduced radiation response in hypoxic tissue, and is the reason that higher radiation doses are used in the postoperative setting when there is diminished microcirculation.
The primary mediator of cell death in response to ionizing radiation, in both tumor and normal tissue, is damage to DNA by indirect ionization by radiation-induced free radicals.2 Indirect DNA damage is characteristic of sparsely ionizing radiation including not only X-rays, but also commonly used charged particles such as electron and proton therapy. In contrast, densely ionizing radiation (e.g., neutrons, αparticles) with a higher linear energy transfer (LET) deposit their energy densely along their incident tracks, and therefore more commonly induce double-strand DNA breaks directly, without the intermediate ionization of cellular water.
The initial deposition of radiation energy in tissue and the resulting DNA damage occur within thousandths of a second of exposure. The biological response to DNA damage includes modulation of cell death, differentiation, and survival pathways, and activation of DNA repair. These biologic processes occur orders of magnitude more slowly than the initial DNA damage. The ultimate cellular response to radiation can be repair, senescence, differentiation, or cell death. The latter may occur via apoptosis, a relatively rapid process, but more commonly occurs as a mitotic cell death. Misrepair of double-stand DNA breaks generates chromosomal abnormalities, and cells die during failed mitosis, often several generations later.
Dose and Fractionation Schedule
The Systeme International d/Unites (SI) unit of radiation dose is the gray (Gy), which is defined as a joule of energy absorbed per kilogram of tissue. An alternate unit of absorbed dose, largely replaced by the gray, is the rad (an acronym for radiation absorbed dose); one gray is equal to 100 rads. Dose is specified to the target volume as defined by the treating radiation oncologist. Most epithelial malignancies are treated to a total dose in the range of 50–80 Gy, lymphoid malignancies typically respond to doses of 15–40 Gy. Select benign conditions can be treated with lower doses; hypertrophic scars and keloids are commonly prescribed doses in the range of 4–20 Gy.
Fractionation refers to the delivery of specified radiation dose in temporally separate treatments, and is recommended to both increase the efficacy of effects on target tissue and to allow normal irradiated tissue to repair radiation damage. Thus, the schedule of radiation fractionation can be used to both increase efficacy of the dose to the target and minimize radiation damage to normal tissue. Common fractionation schemes using conventional radiotherapy deliver treatments at intervals ranging from twice daily to once per week.