Radiation Therapy Following Breast-Conserving Surgery
Laurie W. Cuttino
Frank A. Vicini
Introduction
In 2008, more than 250,000 women were diagnosed with breast cancer, making the disease the most common nonskin malignancy in women (1). Radiation therapy has been used in the treatment of breast cancer for more than a century and was initially used to treat recurrent or residual disease after radical mastectomy. As surgical techniques evolved from radical mastectomy to a more conservative approach, radiation therapy became a critical part of treatment after lumpectomy and has been routinely employed to compensate for the increased risk in local failure with a less aggressive resection.
Over the last several decades, a number of prospective clinical trials have been conducted to demonstrate the equivalence of less extensive surgical techniques to radical mastectomy. After modified radical mastectomy was shown to result in equivalent outcome (2), studies were designed to evaluate the differences in local control and survival with this procedure when compared to breast-conserving surgery and whole-breast radiotherapy. With long-term follow-up, these trials did not demonstrate significant differences in survival with breast conservation (3,4,5,6,7,8). As mastectomy was associated with both physical and psychological morbidity, breast-conserving surgery followed by radiotherapy became the standard of care for the treatment of early-stage breast cancer in the late 1980s.
Just as surgical techniques have changed, radiotherapy techniques have also evolved into highly sophisticated treatments based on a three-dimensional model of the patient’s actual anatomy. Local control with modern surgical and radiotherapy techniques is higher than reported in the original trials investigating breast-conserving treatment. The risk of local recurrence is now thought to be approximately 0.5% per year (9,10,11). With excellent disease control achieved, newer radiotherapy techniques attempt to maximize cosmetic outcome and quality of life. Newer modalities such as accelerated partial breast irradiation (APBI), accelerated whole-breast irradiation, and intensity-modulated radiotherapy (IMRT) are being investigated. This chapter describes the rationale and evidence for the application of radiation therapy after breast-conserving surgery and the techniques used in its delivery.
Invasive Disease
Radical mastectomy, introduced by Halsted and Meyer, was the standard of care for the surgical management of breast cancer for most of the 20th century. In 1971, the National Surgical Adjuvant Breast and Bowel Project (NSABP) initiated the B-04 clinical trial to determine “whether patients with either clinically negative or clinically positive lymph nodes who received local or regional treatments other than radical mastectomy would have outcomes similar to those achieved with radical mastectomy.” A total of 1,079 women with clinically negative axillary nodes were randomized to radical mastectomy or total mastectomy (no axillary dissection) with or without radiation therapy. A total of 586 women with clinically positive axillary lymph nodes were randomized to radical mastectomy or total mastectomy (no axillary dissection) followed by radiation therapy. Although the study was not powered to detect small differences between arms, no statistically significant advantages in either disease-free or overall survival were seen with more aggressive surgery after 20 years of follow-up (2).
After radical surgery was not shown to result in improved outcomes, several groups began to investigate breast-conserving therapy with lumpectomy or quadrantectomy followed by whole-breast irradiation. A number of prospective, randomized trials were initiated to determine whether a breast-conserving approach would be equivalent to mastectomy. The largest U.S. breast conservation trial, NSABP B-06, randomized women to mastectomy, lumpectomy alone, or lumpectomy followed by radiotherapy. After greater than 20 years of follow-up, no significant differences in either local control or survival were observed between patients treated with mastectomy and those treated with lumpectomy and radiotherapy. The results of six modern trials have been published and are shown in Table 19.1. These trials consistently demonstrate no adverse effect on survival with breast conservation versus mastectomy with mature follow-up available (3,4,5,6,7,8).
After the results of these trials became available, the National Institutes of Health held a consensus development conference on the treatment of early-stage breast cancer. The panel concluded that breast-conserving surgery followed by radiotherapy was preferable to mastectomy because it provides equivalent outcome while preserving the breast and reducing physical and psychological morbidity (12).
Several randomized trials have also investigated whether women with early-stage breast cancer can be adequately treated with breast-conserving surgery alone (5,13,14,15,16,17). The long-term results of these trials are summarized in Table 19.2. The NSABP B-06 trial randomized women to mastectomy, lumpectomy, or lumpectomy followed by radiotherapy. Patients treated with lumpectomy alone experienced a significantly higher incidence of local recurrence at 20 years. The addition of radiotherapy decreased the risk of local recurrence after breast-conserving surgery from 39.2% to 14.3% (5). The Milan group randomized 567 women to quadrantectomy with or without radiotherapy. After 10 years of follow-up, radiotherapy decreased the risk of local recurrence from 23.5% to 5.8% (13). The available data from these trials show that positive margins and young age (<35–40 years) are independent risk factors for local recurrence. Local recurrence in younger patients can be significantly reduced with higher radiation doses. There is no
evidence to suggest that patients with lobular histology have a higher risk of local recurrence after breast-conserving surgery and radiotherapy than patients with ductal cancer (18).
evidence to suggest that patients with lobular histology have a higher risk of local recurrence after breast-conserving surgery and radiotherapy than patients with ductal cancer (18).
Table 19.1 Prospective Randomized Trials of Mastectomy Versus Breast-Conserving Treatment for Invasive Breast Cancer | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
These trials clearly demonstrate that the addition of radiotherapy after lumpectomy significantly improves local control. Until recently, however, radiotherapy was not thought to be associated with an improvement in overall survival. In 2005, the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) published the results of a meta-analysis of 42,000 women in 78 randomized treatment comparisons. These patients included 7,300 women treated in ten trials with breast-conserving surgery, with or without radiotherapy. In this analysis, radiotherapy reduced the risk of local recurrence from 26% to 7% at 5 years. Radiotherapy produced similar proportional reductions in local recurrence in all women, irrespective of age or tumor characteristics. Notably, the addition of radiotherapy decreased the 15-year breast cancer mortality risk from 35.9% to 30.5%, with an overall mortality reduction of 5.3%. Essentially, one death from breast cancer would be prevented for every four local recurrences avoided (19). The EBCTCG also reported a small but significant increased risk of contralateral breast cancers, cardiovascular disease, and other cancers with radiotherapy. Although an increase in non–breast cancer mortality was observed, the trials analyzed were conducted before the era of three-dimensionally planned radiotherapy. These risks can be minimized or avoided entirely with modern treatment techniques.
Recent trials have further investigated whether radiotherapy can be safely avoided in highly selected patients using the most modern surgical techniques followed by hormonal therapy. The Cancer and Leukemia Group B randomized 636 women older than age 70 years with T1 N0, estrogen receptor (ER)–positive tumors to lumpectomy and tamoxifen, with or without whole-breast radiotherapy. The addition of radiotherapy improved the rate of local recurrence at 5 years from 4% to 1% (p < 0.001) (20). A Canadian study randomized 769 women older than age 50 years with T1-2 N0, ER-positive tumors to lumpectomy and tamoxifen, with or without whole-breast radiotherapy. Radiotherapy decreased the rate of local recurrence at 5 years from 7.7% to 0.6% (21). Given the importance of local control demonstrated in the EBCTCG meta-analysis, radiotherapy following breast-conserving surgery remains the standard of care in patients with a life expectancy of 5 years or more, even for those with low-risk features.
Table 19.2 Prospective Randomized Trials of Breast-Conserving Surgery With or Without Radiotherapy for Invasive Breast Cancer | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 19.3 Prospective Randomized Trials of Lumpectomy With or Without Radiotherapy for Ductal Carcinoma in Situ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Ductal Carcinoma in Situ
As with invasive disease, ductal carcinoma in situ (DCIS) was initially managed with mastectomy. After the results of the trials investigating breast conservation for invasive disease became available, this approach was investigated for the treatment of DCIS. Four large prospective, randomized trials have been conducted to determine whether patients with DCIS benefit from the addition of radiotherapy after breast-conserving surgery. The results of these trials consistently show significant reductions in local recurrence with radiotherapy and are summarized in Table 19.3. In the NSABP B-17 trial, 818 patients were randomized to breast-conserving surgery versus surgery plus radiotherapy. The 12-year actuarial risk of local failure was 15.7% for patients treated with radiotherapy, compared with 31.7% for patients treated with surgery alone (22,23). The European Organization for Research and Treatment of Cancer (EORTC) randomized 1,002 patients to the same schema. Radiotherapy reduced the risk of local failure at 10 years from 26.4% to 15.0% (24). The United Kingdom Coordinating Committee on Cancer Research conducted a trial designed with a 2 × 2 factorial plan to assess the effects of both radiotherapy and tamoxifen after lumpectomy. At a median follow-up of 52.6 months, 1,030 patients were analyzed to determine the effect of radiotherapy. Radiotherapy reduced the rate of local failure from 14% to 6.0% (without the addition of tamoxifen) (25). In a Swedish trial, 1,046 patients were randomized to resection with or without radiotherapy. At a median follow-up of 5.2 years, radiotherapy reduced the rate of local failure from 22.5% to 8.3% (26). A recent meta-analysis of these four randomized trials reported that the addition of radiotherapy reduces the risk of local recurrence by approximately 60% (27). Although no trial was sufficiently powered to demonstrate a significant improvement in overall survival, the three trials that reported event-free or disease-free survival all consistently showed a significant benefit from radiotherapy (24,26,28).
Some authors have argued that radiotherapy is unnecessary in patients some patients with DCIS. In 1996, investigators from the University of Southern California introduced the Van Nuys Prognostic Index. This was a retrospective analysis of 333 patients with DCIS treated with breast-conserving surgery (195 by excision only and 138 by excision followed by radiotherapy). Pathologic evaluation of surgical specimens was particularly rigorous, with tissue sectioned in 2-to 3-mm intervals and processed in sequence. Multivariate analysis showed tumor size, margin width, and pathologic classification to be predictors of recurrence. This report concluded that patients with low-grade tumors ≤15 mm without necrosis resected with at least a 10-mm surgical margin can be treated with excision alone (29). The index was updated in 2003 to include age, with patients >60 years old considered at low risk of recurrence (30). Since its introduction, several authors have attempted to independently validate the Van Nuys Prognostic Index. Although some series suggest that radiotherapy can be avoided in low-risk patients, multiple series have not shown the index to be a reliable predictor of local recurrence (31,32).
Investigators at the Dana-Farber/Harvard Cancer Center attempted to prospectively determine whether radiotherapy can be omitted in patients with small, low-grade tumors resected with at least 10-mm surgical margins. In 2002, the study was closed to accrual after an interim analysis found that was unexpectedly high. After a median follow-up of 40 months, 158 patients had been enrolled. The rate of ipsilateral local recurrence was 2.4% per patient-year, with a 5-year rate of 12%. Of the local recurrences, 31% were invasive (33). At present, no prospective study has identified a low-risk population of patients in whom radiotherapy can be safely avoided. As such, postoperative radiotherapy after lumpectomy remains the standard of care for most patients with DCIS.
Whole-Breast Radiotherapy Techniques
The target for standard postoperative radiotherapy is the entire ipsilateral breast. In general, the breast is treated with megavoltage
photons generated by a linear accelerator using opposed tangential fields that encompass the entirety of the breast, chest wall, and low axilla. If the regional lymph node chains are at high risk for harboring microscopic disease, additional or modified fields are used to encompass these areas. The treatment planning process begins with a reproducible immobilization of the patient. Some centers employ a custom mold of the patient’s body, while others use a special apparatus (breast board) that is placed on top of the treatment couch, allowing reproducible positioning of the breast and abduction of the ipsilateral arm. A typical patient setup is shown in Figure 19.1. A computed tomography (CT) scan is then performed in the treatment position. This CT data set is then imported into the radiation treatment planning system. Essentially, the planning system constructs a three-dimensional model of the patient’s body, allowing the radiation oncologist to accurately target the breast (and regional lymph nodes if required) while minimizing dose to the ipsilateral lung. This process is known as three-dimensional conformal radiotherapy (3D-CRT) and allows the contralateral breast and heart to be avoided entirely in most patients.
photons generated by a linear accelerator using opposed tangential fields that encompass the entirety of the breast, chest wall, and low axilla. If the regional lymph node chains are at high risk for harboring microscopic disease, additional or modified fields are used to encompass these areas. The treatment planning process begins with a reproducible immobilization of the patient. Some centers employ a custom mold of the patient’s body, while others use a special apparatus (breast board) that is placed on top of the treatment couch, allowing reproducible positioning of the breast and abduction of the ipsilateral arm. A typical patient setup is shown in Figure 19.1. A computed tomography (CT) scan is then performed in the treatment position. This CT data set is then imported into the radiation treatment planning system. Essentially, the planning system constructs a three-dimensional model of the patient’s body, allowing the radiation oncologist to accurately target the breast (and regional lymph nodes if required) while minimizing dose to the ipsilateral lung. This process is known as three-dimensional conformal radiotherapy (3D-CRT) and allows the contralateral breast and heart to be avoided entirely in most patients.
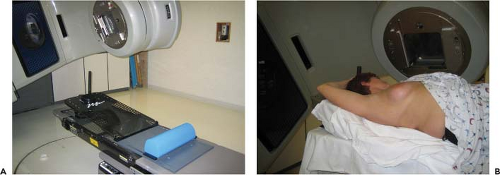 Figure 19.1. A patient immobilization device (A) and patient setup (B).
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|





