8 Principles of Osteosynthesis Abstract Bone tissue is derived from various embryological lineages which follow a process of either intramembranous or endochondral ossification. Bone is formed of organic and inorganic fractions composed of cells, proteins, and minerals that provide its physiological and structural characteristics. The organization of intrinsic collagen fibers determines whether bone tissue matures to become woven or lamellar. Bone consolidation can be direct or indirect. The latter occurs through five overlapping phases of callus formation. Fracture treatment should include reduction, absolute or relative stabilization, fixation of the fragments, and rehabilitation. However, fracture treatment is not exempt from complications, such as infection, delayed union, nonunion, and malunion. Distraction osteogenesis is a procedure that uses the process of generating new bone tissue between osteotomized bone fragments and can be used when other forms of bone fracture treatment or congenital malformations fail to provide enough bone length. This chapter presents the current knowledge of bone microanatomy and fracture pathophysiology, which constitute the foundations for bone healing and synthesis, and gives a comprehensive review of the most commonly used surgical techniques for fracture fixation and its complications. Keywords: bone microanatomy, distraction osteogenesis, fracture stabilization, ossification, osteomyelitis, osteosynthesis devices, pseudarthrosis Osteogenesis is the process of bone formation. The skeletal components are derived from three embryological lineages. Neural crest cells give rise to the pharyngeal arches and the axial skeleton; paraxial mesoderm forms the craniofacial skeleton and most of the axial skeleton through the division of the somites; and finally, the limbs’ skeleton develops from the lateral plate mesoderm. Differentiation of mesenchymal tissue into bone can follow an intramembranous or an endochondral mechanism. Intramembranous ossification is characterized by direct bone formation in a connective tissue membrane in the absence of cartilage. It occurs in flat bones, in the fracture callus, and in distraction osteogenesis. Endochondral ossification occurs through a cartilage intermediate, called the primary ossification core in the embryonic period, and a secondary ossification core after birth. This type of ossification occurs in long bones and in the fracture callus as well. Bone tissue consists of two essential components, namely extracellular matrix (ECM) and cells. The ECM contains an organic phase and a mineral fraction. The cells constitute a small percentage included in the organic fraction of bone tissue. The organic fraction of the ECM constitutes 30% of dry bone mass and is mainly formed by three protein groups: collagenous, noncollagenous, and glycoproteins. Collagenous proteins form the osteoid, which is the chief element of bone matrix, and provide elasticity and flexibility. Collagen type I is organized in a fibrillar form and has a mineralization potential, providing bone elasticity and tensile strength. Hydroxyproline is a major component of collagenous proteins, and its increased urinary levels reflect bone resorption. Noncollagenous proteins include osteocalcin, a linear peptide hormone produced by osteoblasts during bone formation and incorporated into the bone matrix, and bone morphogenetic proteins (BMPs), which are involved in the formation of new bone, cartilage, and connective tissue. Finally, proteoglycans and glycoproteins form the fundamental amorphous substance that surrounds cells and collagen. They are composed mainly of hyaluronic acid chains bounded to subunits of chondroitin sulfate and keratin sulfate. The inorganic or mineral phase accounts for 50–70% of bone composition and is responsible for providing mechanical rigidity and strength to the ECM. It is formed by hydroxyapatite crystals consisting of 80% tricalcium phosphate and 10% calcium carbonate. Different growth factors, such as the Wnt family and BMPs, stimulate mesenchymal stem cell differentiation into osteoblastic progenitors, such as pro-osteoblasts, which then become osteoblasts and ultimately osteocytes. These cells are all located in the endosteal and periosteal layers. Osteoblasts are found on the surface of bone, and their main function is osteoid production. They also synthesize alkaline phosphatase and osteocalcin and have receptors for parathyroid hormone (PTH), 1,25(OH)2-vitamin-D, glucocorticoids, prostaglandins, and estrogens. Osteocytes are found within the bone matrix and constitute 90–95% of the cellular component. They develop from the osteoblasts located in the lacunae and are characterized by large cytoplasmic processes that extend radially through the osteons and to the canaliculi, allowing communication with other osteocytes and osteoblasts. Osteocytes are key in regulating homeostasis of extracellular calcium and phosphorus. Osteoclasts are located in the trabecular bone surface in spaces called Howship’s lacunae and at the head of the cortical perforating cones. They are activated by osteoblasts to exert their main function, which is to lyse bone. The contact zone of their cell membrane has a hairy pattern that increases the area of resorption. Once in contact with the bone surface, the osteoclast’s cell membrane delimits an area fixed and sealed by integrins, where pH is reduced through a proton pump, and acid proteases are released, dissolving the mineral matrix and degrading the collagen component. Depending on the organization of collagen fibers, two types of bone can be identified: woven and lamellar. Woven bone owes its name to its characteristically irregular organization of cells and collagen fibers, which, together with its increased water content, make it highly deformable and flexible. Woven bone is the primary or immature bone found in the skeletons of embryos and newborns, and it is gradually replaced by mature (e.g., lamellar) bone during skeletal growth. In adults woven bone may persist as part of the ear bones, tendon and ligament insertions, and cranial bone sutures. In addition, woven bone is the first to appear in the callus of healing fractures. Lamellar bone, also called secondary or mature bone, completely replaces woven bone by 4 years of age and is organized in a specific and regular pattern according to the supported loads. Cortical bone is a type of lamellar bone forming 80% of an adult’s skeleton. It is formed by a set of elementary functional units called osteons, composed of a series of concentrically arranged bone plates. At the center of each osteon, blood and lymphatic vessels and nerves run through the haversian canals, which interconnect with each other and with the periosteum through Volkmann’s canals. The spaces between the osteons are occupied by interstitial lamellar systems ( The periosteum is a well-vascularized layer that covers the outermost part of cortical bone and is divided into an outer sheet, composed of poorly active fibrous tissue; and an active inner layer or cambium layer, lying in direct contact with the cortical bone surface and rich in osteoblast cells, which orchestrate callus formation and repair of bone following fracture. Cancellous bone forms 20% of the skeleton and consists of a set of bony trabeculae arranged in a randomly oriented three-dimensional network that provides load support. The spaces between the trabeculae are occupied by bone marrow and fatty tissue. Cancellous bone has lower rigidity than cortical bone, but a metabolic activity that is eight times higher ( Note The cambium layer is rich in osteoblasts. It activates after a fracture and forms the fracture callus. A fractured bone can heal or consolidate directly or indirectly depending on fracture treatment and stability. Direct, primary, or cortical consolidation takes place in anatomically reduced fractures with absolute stability. It is produced by the passage of vessels through the bone contact areas and osteoblastic apposition of new bone in areas of no contact. Direct consolidation occurs without callus formation. Indirect or secondary consolidation occurs in fractures treated with flexible fracture stabilization, which allows interfragmentary mobility. Indirect consolidation occurs through callus formation along a set of five overlapping phases, namely hematoma, inflammation and angiogenesis, reparative, ossification, and remodeling. In the first phase of hematoma, the blood clot releases interleukins-1 and -6 (IL-1 and IL-6) and tumor necrosis factor-α (TNF-α) that trigger the inflammation cascade. The inflammatory and angiogenesis phase then ensues with neutrophils, macrophages, and lymphocytes being recruited to the site of injury to remove debris and initiate repair. During this phase the initial hematoma is gradually replaced by a fibrin clot, and osteoclasts begin resorption of the bony ends. Vasodilation and hyperemia of the surrounding soft tissues promote the formation of new capillaries that grow through the site of injury, and precursor cells begin to proliferate and differentiate into osteoblasts. Later in this phase, fibrin, collagen, and reticular fibers are replaced by granulation tissue. The reparative phase starts at the offset of inflammation and is characterized by the formation of a soft callus composed mainly of cartilage and varying amounts of connective tissue and blood vessels, in response to mechanical and biological factors. The ossification phase starts at the third week to form the hard callus and lasts between 12 and 16 weeks, until the bony ends are firmly joined together. During this phase, the soft callus undergoes endochondral and intramembranous ossification. Finally, in the remodeling phase the immature woven bone with its irregular microstrucure is gradually replaced by mature, laminar, anisotropic bone in response to the mechanical load ( Fig. 8.1 Bone microanatomy. (Reproduced from Schuenke, Schulte, and Schumacher, Atlas of Anatomy, 2nd edition, ©2014, Thieme Publishers, New York. Illustration by Karl Wesker/Markus Voll.) Fig. 8.2 Stages of bone callus formation during secondary fracture healing. (a) Hematoma filling the fracture gap. (b) Granulation tissue and connective tissue replacing the hematoma in the fracture gap with ingrowth of blood vessels. (c) Fibrocartilage replacing the connective tissue in the fracture gap. (d) Woven bone replaced by lamellar bone through haversian remodeling. (Reproduced from Ehrenfeld, Manson, Prein, Principles of Internal Fixation of the Craniomaxillofacial Skeleton Trauma and Orthognathic Surgery, ©2012, Thieme Publishers, New York.) Note Bone callus is formed through five established phases: hematoma, inflammatory and angiogenesis, reparative, ossification, and remodeling. The objective of fracture treatment is to promote consolidation and restore the bone’s mechanical properties for maximum functional recovery. Different factors affect the process of bone regeneration, such as age, genetics, and mechanical factors. Anatomical relationships between bone fragments can be restored by open or closed manipulation. The aim of anatomical reduction is to correct shortening, angulation, and rotation. Fracture stabilization can be achieved by conservative (e.g., plasters) or surgical treatment. The objective is to reduce the mobility of the bony ends to allow bone healing. External fixators are not aimed at providing definitive stabilization, but rather a reasonable reduction until other issues are solved (e.g., vascular injury or soft tissue reconstruction). The biological response and the type of consolidation occurring at the fracture site are highly dependent on the degree of relative or absolute stabilization yielded by the selected treatment. For example, fractures stabilized with a plaster or elastic fixation (e.g., Kirschner wires), undergo secondary consolidation. Relative stability allows microscopic movements between the fragments following load application. Techniques of relative stability include intramedullary nailing, which controls fracture angulation and displacement, but not rotation. The addition of locking screws (e.g., locked intramedullary nailing) can help to correct shortening and rotation. Bridging plates fixed with locked screws provide relative stability by bridging the area of greater comminution while maintaining length and alignment. Finally, external fixators can correct bone alignment, but the stiffness of the frame is limited because the rods are fixed to pins several millimeters or even centimeters above the fracture ( Fig. 8.3 (a) Comminuted fracture of the proximal phalanx of the thumb and second metacarpal. (b) Thumb fixation with Kirschner wires and second metacarpal fixation with 2-mm bridging plate. Absolute stability is achieved with rigid fixation by interfragmentary compression at the fracture site resulting in primary or direct consolidation. Techniques of absolute stability include compression screws or plates that transform rotational forces into linear fracture compression, and tension bands that convert distraction tensile forces into compression forces at the fracture site. Note Absolute stability results in primary consolidation, whereas relative stability leads to fracture healing through callus formation (e.g., secondary consolidation). Kirschner wires are available in different diameters, from 0.8 to 3 mm. They are versatile and can be used in any small bone fracture, but comminuted fractures are their primary indication. Wires can be inserted through a closed or open approach, causing minimal trauma to the surrounding tissues, and they may be used as temporary or definitive fixation providing relative stability to the fracture. One of their limitations is that they do not allow interfragmentary compression. Screws are helical devices that convert rotational forces into linear motion ( Screws can also be self-tapping or self-drilling. Self-tapping screws have a taplike flute in their leading threads, which allows them to tap their own hole as they are driven into, but need bone drilling prior to insertion. Self-tapping screws are not recommended for use as lag screws. Self-drilling screws have a preliminary drill-like fluted tip that allows insertion without predrilling of bone ( Lag or compression screws provide reduction between two fragments and achieve absolute stability. The principle of lag screws is based on making the screw glide without purchase through the proximal fragment and thread through the distal fragment so that, with each turn, the distal fragment is brought against the proximal fragment. This can be obtained either with partially threaded screws or by overdrilling the near cortex (
8.1 Basic Science
8.1.1 Mechanisms of Ossification
8.1.2 Bone Composition
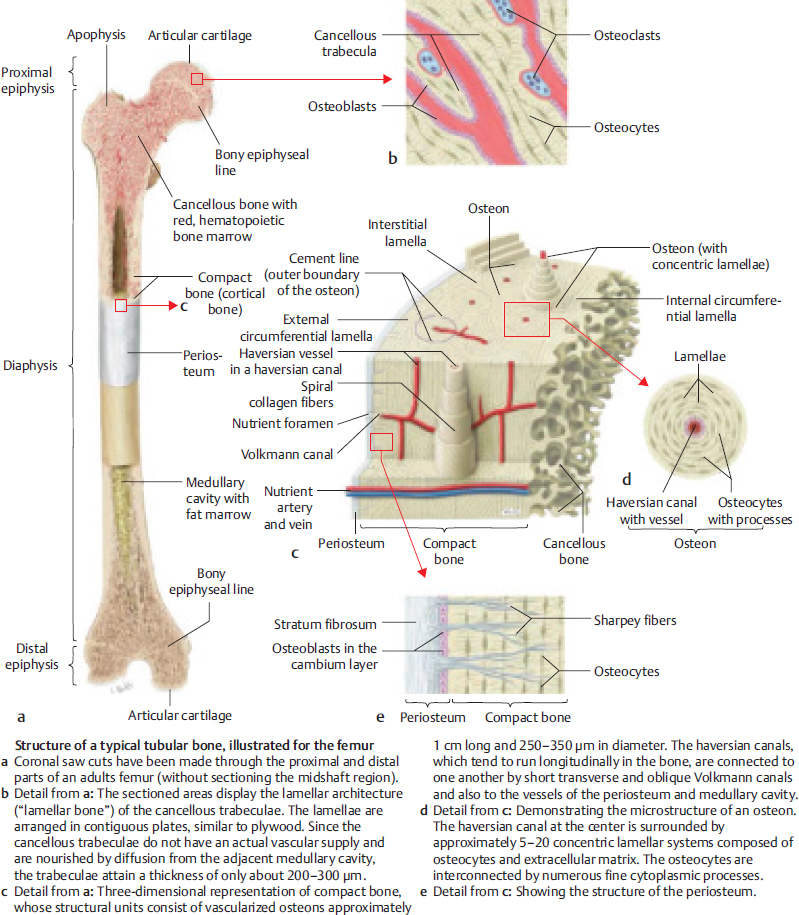
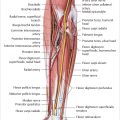
8.1.3 Bone Microanatomy
 Fig. 8.1).
Fig. 8.1).
 Fig. 8.1).
Fig. 8.1).
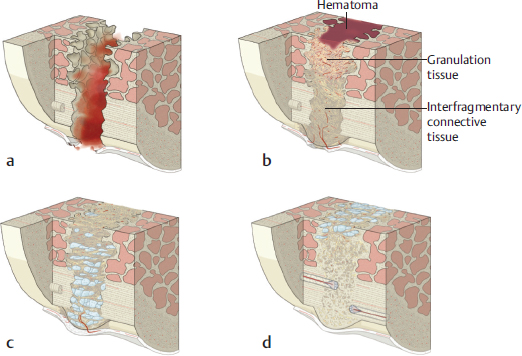
8.1.4 Bone Healing
 Fig. 8.2). According to Wolff’s law, bones adapt to the load they are submitted to, so that with increasing loads, bones become thicker and stronger, whereas in situations of deceased loads, they become weaker and less dense.
Fig. 8.2). According to Wolff’s law, bones adapt to the load they are submitted to, so that with increasing loads, bones become thicker and stronger, whereas in situations of deceased loads, they become weaker and less dense.
8.2 Fracture Repair
8.2.1 Reduction
8.2.2 Stabilization
Types of Stability
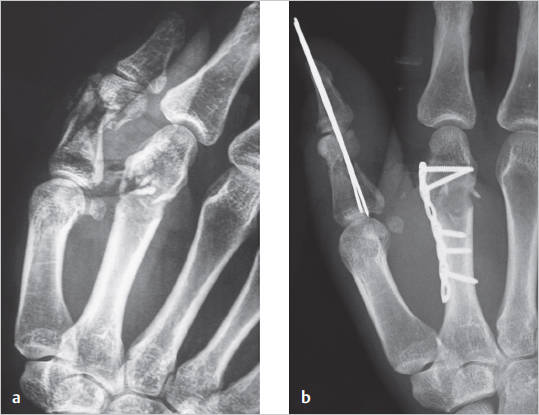
 Fig. 8.3).
Fig. 8.3).
8.2.3 Fixation
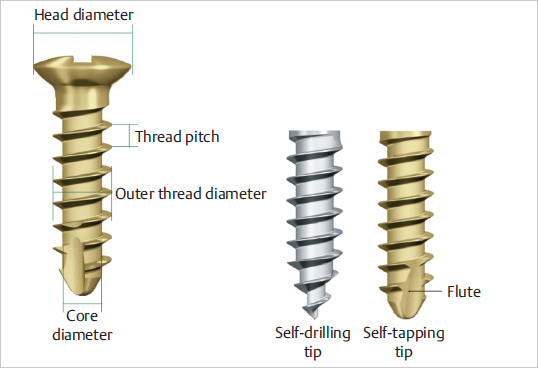
Osteosynthesis Devices
 Fig. 8.4). Cortical screws are designed to penetrate through the rigid and hard structure of cortical bone. They have a narrow thread pitch with closely spaced threads. Cancellous screws are designed to fix epiphyseal and metaphyseal cancellous bone and osteoporotic bone. The threads are more separated so that with each 360-degree turn the screw advances a longer distance. In addition, the thread (external) diameter is comparatively larger than the inner (core) diameter.
Fig. 8.4). Cortical screws are designed to penetrate through the rigid and hard structure of cortical bone. They have a narrow thread pitch with closely spaced threads. Cancellous screws are designed to fix epiphyseal and metaphyseal cancellous bone and osteoporotic bone. The threads are more separated so that with each 360-degree turn the screw advances a longer distance. In addition, the thread (external) diameter is comparatively larger than the inner (core) diameter.
 Fig. 8.4). Some self-tapping screws are also self-drilling.
Fig. 8.4). Some self-tapping screws are also self-drilling.
 Fig. 8.5). It is very important to place the screw perpendicular to the fracture line; otherwise a shear plane can preclude proper reduction. Generally a screw should be tightened to only two-thirds of its strength to allow resistance to any additional functional loading. Compression screws are available in diameters from 1 to 2.7 mm. They are best used in long spiral fractures, and their use in short oblique fractures is ill advised. Cannulated compression screws are a variable-pitch type of headless implant that are placed within the bone by using a thin guidewire. These screws are tapered with a differential thread pitch to increase compression as the screw is advanced (
Fig. 8.5). It is very important to place the screw perpendicular to the fracture line; otherwise a shear plane can preclude proper reduction. Generally a screw should be tightened to only two-thirds of its strength to allow resistance to any additional functional loading. Compression screws are available in diameters from 1 to 2.7 mm. They are best used in long spiral fractures, and their use in short oblique fractures is ill advised. Cannulated compression screws are a variable-pitch type of headless implant that are placed within the bone by using a thin guidewire. These screws are tapered with a differential thread pitch to increase compression as the screw is advanced ( Fig. 8.6). Their main use includes scaphoid fracture fixation, but they can also be used in joint arthrodesis and small tubular bone nonunions.
Fig. 8.6). Their main use includes scaphoid fracture fixation, but they can also be used in joint arthrodesis and small tubular bone nonunions.
Plastic Surgery Key
Fastest Plastic Surgery & Dermatology Insight Engine