12 Principles of cancer management
Synopsis
 There has been a gradual progression from radical to more conservative resections of many cancers.
There has been a gradual progression from radical to more conservative resections of many cancers.
 Neoadjuvant therapies have facilitated resectability and even down-staging of the disease.
Neoadjuvant therapies have facilitated resectability and even down-staging of the disease.
 A better understanding of immunology has led to the development of biologic therapies beyond the standard cytotoxic chemotherapies.
A better understanding of immunology has led to the development of biologic therapies beyond the standard cytotoxic chemotherapies.
 Tailored immunotherapies are being studied and represent a new paradigm in the treatment of cancer.
Tailored immunotherapies are being studied and represent a new paradigm in the treatment of cancer.
History of cancer treatment
Neoadjuvant treatment is the term used to describe either chemotherapy and/or radiation that are administered prior to tumor excision. Neoadjuvant therapy is used to shrink unresectable tumors rendering them resectable, or to decrease the magnitude of the ablation necessary to achieve negative margins.1 Patients may undergo a complete response with total preoperative tumor eradication, or a partial response to neoadjuvant therapy. The response to neoadjuvant chemotherapy is an important predictor of outcome and can determine which chemotherapy is used postoperatively.2 Clinically neoadjuvant therapy is a common part of treatment algorithms for many solid tumors. For example, randomized trials demonstrate lower recurrence rates for T3 and T4 rectal tumors when chemoradiation is delivered preoperatively as compared to postoperatively.3 Furthermore, for distal tumors lying near the anus, neoadjuvant therapy facilitates sphincter preservation by converting cases which potentially require abdominoperineal resection to low anterior resections.4
Disadvantages of neoadjuvant therapy include the possibility for disease progression prior to surgical resection and increased rates of surgical complications due to side-effects of chemoradiotherapy. For example, in a review of nearly 1200 microsurgical breast reconstructions, Mehrara et al. demonstrated that neoadjuvant chemotherapy was an independent risk factor for postoperative complications in breast cancer patients undergoing microvascular breast reconstruction resulting in increased rates of wound complications if surgery was performed less than 6 weeks after completion of chemotherapy.5
In the adjuvant or postablative setting, chemotherapy or radiation is implemented to decrease locoregional recurrence or systemic disease by treating microscopic tumor deposits or metastases that are not detectable at the time of surgery. It is important to emphasize, however, that even if radiation or chemotherapy is planned postoperatively, all attempts should be made (if surgically feasible) to obtain microscopically negative tumor margins at the time of surgical resection. This concept is based on the fact that negative tumor margins are an important predictor of local or regional metastases in most solid tumors.6
Surgery
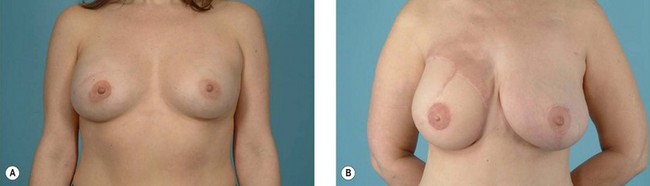
The earliest treatment for tumors was fulguration using electrocautery, application of toxic materials, or surgical extirpation. Due to the high local recurrence rates seen after tumor excision, surgical management at the turn of the 20th century developed into a “bigger is better” approach with wide margins. The best-known example is the Halsted radical mastectomy for invasive breast cancer advocating the removal of the pectoralis major muscle, all of the breast tissue and axillary nodes.7 Similarly, head and neck malignancies were treated with internal jugular vein ligation, sternocleidomastoid muscle resection, and spinal accessory nerve sacrifice. Such aggressive approaches to tumor control have subsequently been shown to be unnecessary for many cancers. Instead, the efficacy of limited anatomic resections has been proven in studies which: (1) demonstrate equivalency with more radical approaches, or (2) combine surgery with multimodality therapy. For example, margins greater than 2 cm have shown no benefit in the treatment of cutaneous melanoma.8 Additionally, effective treatment of breast cancer no longer mandates mastectomy if lumpectomy is combined with postoperative radiotherapy.9 Modern surgical therapy uses insight gained from clinical trials to minimize the scope of resection with an eye on preserving form and function (Fig. 12.1). Radical approaches are reserved for only the most advanced cancers with extensive local invasion.
While the utility of primary tumor excision has been established, the role of surgery in management of metastatic disease is less clear. The majority of patients with metastasis receive palliative treatment with radiation to achieve local control and chemotherapy for disseminated disease. However, if thorough evaluation reveals isolated or a limited number of metastases in a patient with a good prognosis, both cure and extended survival have been achieved with surgical “metastectomies.” Patients with extremity sarcomas who undergo pulmonary resections have 5-year survival rates up to 25%.10 Similarly, hepatic lobectomy for colorectal metastasis is associated with 25–40% 5-year survivorship in selected patients.11
Radiation
Ionizing radiation is defined as energy sufficient to release electron(s) from an atom or molecule. Most radiation agents use either photons or electrons to cause ionization. The quantity of ionization produced in air by a beam of radiation is termed the exposure and is measured in units of roentgens. The more clinically relevant measure is the absorbed dose, which is a calculation of the amount of energy absorbed per unit mass. This is quantified in joules per kilogram, or gray units (Gy). Upon entering tissues, the ionizing agent dose increases, but then degrades exponentially as its distance from the source increases in accordance with the inverse-square law.12
The mechanism whereby ionizing radiation achieves tumor cell kill is not completely understood. Ionizing radiation causes cellular damage directly through release of electrons or indirectly by producing free radicals from water. Since oxygen increases the half-life of reactive radical species, radiation is most effective in oxygen-rich environments. When electrons or free radicals come into contact with biologically important molecules, such as DNA, detrimental effects occur. Although cells efficiently repair single-strand DNA breaks, double-strand breaks are less easily handled by cellular machinery. If sufficient DNA damage accumulates prior to the next mitotic cycle the cell is overwhelmed, leading to cell killing. When the interval between successive doses of radiation is increased, greater numbers of cells can repair DNA strand breaks. This principle is termed sublethal damage and provides the rationale for radiation delivery in fractionated daily doses ranging from 1.8 to 2.5 Gy. The success of radiation therapy is predicated on the fact that normal cells have a greater ability to repair sublethal damage between radiation fractions than tumor cells. Radiation therapy is most commonly delivered at a distance from the tumor called external-beam radiotherapy or teletherapy; however, it can also be administered as brachytherapy with high doses given directly into a specified area.12
Clinical use of radiation therapy began in the 1940s and 1950s after early pioneers in the field were able to minimize local complications such as radiation burns and secondary skin cancers. Although uncommon, radiation can be used as monotherapy for radiosensitive tumors such as Hodgkin’s disease.13 For other tumor types equivalency between surgical excision and radiation therapy has been demonstrated. For example, treatment of T1/T2 prostate cancer with either radical prostatectomy or external-beam radiotherapy has comparable survivorship, but with different risk profiles for each therapy.14,15 Choice of treatment is based on patient preference and surgical risk factors as assessed by the treating urologist. Similarly, aerodigestive tumors less than 2 cm respond equally well to either radiation or surgery. In the case of laryngeal neoplasms, radiotherapy is preferred due to its ability for organ preservation.16
Surgeons most commonly work with radiation as part of neoadjuvant or adjuvant regimens in combination with extirpation for solid tumors. These two modalities complement each other to improve locoregional control. While surgery fails at tumor margins where cell counts are low or submicroscopic, radiation works best at the periphery where oxygenation levels are highest. Radiation is least effective in the avascular center of necrotic tumor masses where oxygen tension is poor.17
The increased role of radiation therapy in cancer management has led to refinements in its delivery. In the past radiation was administered in the neoadjuvant setting with the goal of rendering microscopic tumor deposits, spilled inadvertently at the time of resection, incapable of cell division. However, technical difficulties and increased complication rates, such as wound breakdown and infection associated with this schedule of radiation, have led to its preferred use in the adjuvant setting for many cancers. Although the increased rates of wound-healing complications following surgery in irradiated tissues have been thought to be related to the harmful effects of radiation on the local blood supply, the exact cause(s) of this increased risk remains unknown.18 In fact, studies have suggested that radiation injury causes depletion of local tissue stem cells and that restitution of these cells with stem cell injections can improve tissue healing.19
Chemotherapy
The goals of chemotherapy are similar to those of radiation, namely preferentially kill cancer cells relative to normal tissues while minimizing toxicity. The success of chemotherapy is due to the fact that normal cells have improved ability to repair damaged DNA and survive relative to cancer cells. For chemotherapy to be successful, complete eradication of all tumor cells is essential. Agents that kill 99.99% of 109 tumor cells, the approximate number of cells in a 1-cm mass, allow a tumor burden of 105 cells to remain. Since agents are unlikely to kill every tumor cell in a single administration, chemotherapies are delivered in several rounds to achieve maximal cell killing. Administration of chemotherapy in fractions takes advantage of the fact that some agents are effective only in certain stages of the cell cycle (termed kinetic resistance) and minimizes toxicity at each time point by allowing decreased dosing.20 Furthermore, combination chemotherapy using agents with different mechanisms of action and toxicities allows maximal tumor killing while minimizing host side-effects. Recognition of chemotherapy toxicity has led to modifications such as regional administration which allows for increased drug doses with reduced systemic side-effects. Examples of this application include isolated limb perfusion for patients with melanoma in-transit metastases or hepatic artery infusion pumps for colorectal liver metastasis.21,22
Chemotherapeutic agents such as nitrogen mustard and methotrexate were first introduced in the 1940s with the intention of avoiding surgery altogether. While effectiveness of chemotherapeutics as single-modality treatment for hematologic malignancies such as leukemia and lymphoma has been demonstrated, their efficacy in primary treatment of solid tumors has not been borne out in most cases. Exceptions include chemotherapy for subtypes of testicular cancer and combined chemoradiotherapy such as the Nigro protocol for anal cancer.23,24 A common indication for chemotherapy in the management of solid tumors is for palliation of patients with metastatic disease who are ineligible for curative surgery. In such cases chemotherapy is administered with the intent of prolonging survival and improving quality of life.
Surgeons most frequently encounter chemotherapy as part of neoadjuvant or adjuvant treatment in combination with tumor excision. Examples of tumors commonly treated in this manner include breast, colon, and head and neck cancers. Synergy between surgery and chemotherapy is exemplified in the case of extremity sarcomas. Previously patients with osteosarcomas were treated with amputation; however, the introduction of neoadjuvant chemotherapy has increased rates of limb preservation with equivalent long-term survival.25
Immunotherapy and biologics
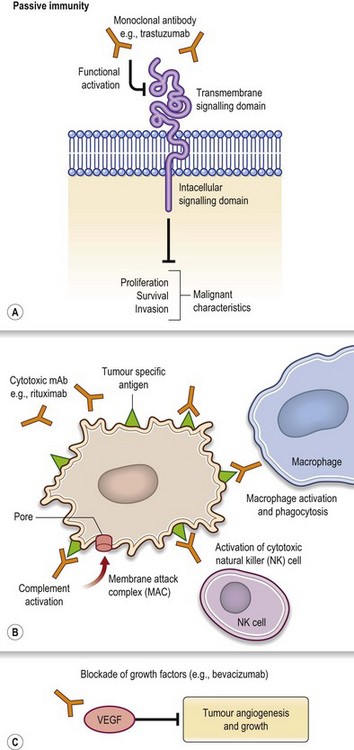
Passive and active immunotherapy are newer forms of cancer treatment which use immune mechanisms to kill tumor cells. In passive immunotherapy the host immune system remains quiescent while therapeutic agents are introduced to eradicate tumor cells. The most common application of this treatment is delivery of monoclonal antibodies against specific tumor antigens (Fig. 12.2). Advantages of this approach are the highly specific nature of therapy which theoretically decreases both side-effects and toxicity. A widely used agent is trastuzumab, a monoclonal antibody that binds to the extracellular segment of the HER2/neu receptor, overexpressed in approximately 25% of breast cancers.26,27
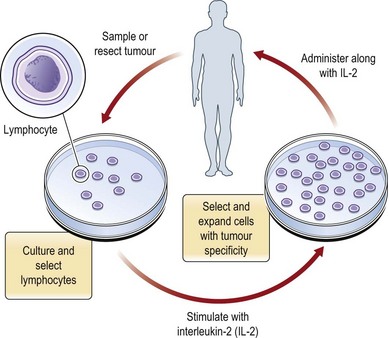
Adoptive immunotherapy is an alternative form of passive immunotherapy wherein cells with antitumor activity are introduced into the host (Fig. 12.3). An example would be ex vivo manipulation of host autologous tumor-infiltrating lymphocytes, which have a naturally occurring reactivity to cancer, in the presence of IL-2 to increase cytolytic potential or tumor antigen recognition.28 Studies are currently evaluating the utility of this therapy for the treatment of melanoma.29
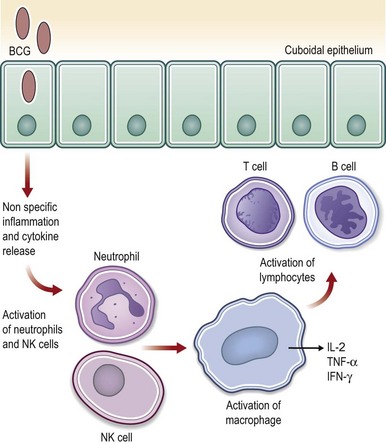
Active immunotherapy describes protocols in which the host immune system is directly stimulated by presentation of antigenic materials. If the antigenic material causes generalized stimulation of the immune system, it is considered nonspecific stimulation (Fig. 12.4). The best-known immunostimulant agent is bacillus Calmette-Guérin (BCG), an attenuated form of bovine tuberculosis bacillus. Although its mechanism of action is unclear, it has proven benefit in the treatment of superficial bladder cancers, with less promising results as a therapy for melanoma.30 Infusions with cytokines are another nonspecific form of active immunotherapy. For example, melanoma is a frequent target for agents such as interleukin-2 and interferon-alpha because it is the tumor with the most immunogenic response.31,32