div class=”ChapterContextInformation”>
5. Clinical Presentation and Diagnostic Evaluation of Male Urethral Stricture
Keywords
Urethral stricture/diagnosisUrethra/diagnostic imagingUrethroplastyCystoscopyPatient reported outcome measuresUrethral diseasesUrethrogramTomographyX-ray computedMagnetic resonance imagingUltrasonographyQuality of life5.1 Introduction
Over the last century, urethral stricture (USx) management has evolved dramatically from the ancient practice of urethral dilation to more complex urethroplasty techniques [1]. While surgical decision making can be complicated the majority of diagnostic tools have remained mostly unchanged for a long period of time [2, 3].
The aim of this chapter is to demonstrate the variation in clinical presentation, diagnostic tests and future possibilities for both preoperative diagnosis and postoperative follow-up of USx.
5.2 Non-invasive Tests: Initial Assessment and Follow-up After Reconstruction
There is a relative lack of evidence regarding the primary presentation of urethral stricture (USx). Structured assessment by means of different Patient-Reported Outcome Measures (PROMs) has predominantly focused on follow-up after urethroplasty, thus, data about primary diagnosis is currently lacking.
5.2.1 Clinical Presentation
In 2012, Rourke et al. [4] described initial complaints and other associated symptoms among 611 patients presenting to a urology clinic with anterior USx. Overall, the most frequent associated symptoms were lower urinary tract symptoms (LUTS), acute urinary retention (AUR), genitourinary pain, and urinary tract infection (UTI), at 92.9%, 29.8%, 22.9% and 20.3%, respectively. The primary presenting complaint were LUTS in 54.3% of the total, followed by AUR and UTI in 23.4% and 6.1%, respectively. Difficult catheterization, gross hematuria, genitourinary pain, urethral abscess/necrotizing fasciitis, renal failure/hydronephrosis, incontinence and sexual dysfunction were present in less than 10% of the cases each. Remarkably, 7.4% of patients had potentially life-threatening complications secondary to anterior stricture. Other complications such as urethral discharge, stones, prostatitis, epididymitis, urethral diverticulum, urethrocutaneous fistula, and infected sinuses are not uncommon [5, 6]. It should also be noted that approximately 60% of cases with urethral cancer may present with USx at some point in their disease course [5]. In any patient with an atypical presentation of USx one should consider urethral cancer in the differential diagnosis.
While most strictures are idiopathic, they are also frequently identified in patients presenting with recurrent UTI, ejaculatory dysfunction, lichen sclerosus (LS), hypospadias, or genital trauma, either internally (previous urethral catheterization, TURP, radical prostatectomy) or externally (straddle trauma, pelvic fracture, prior pelvic surgery). When a detailed history is obtained, up to 62.9% of all patients will have an identifiable cause of their stricture [7–9].
5.2.2 Physical Examination
Physical examination is an important aspect of assessment. In patients with a suspected stricture body habitus in general should be noted for any potential difficulties in lithotomy positioning during future procedures. On abdominal examination the bladder may be palpable during abdominal examination, while the presence of a suprapubic catheter (SPC) can facilitate antegrade assessment of the urethra proximal to the stricture if necessary in the setting of completely obliterated strictures [10]. Examination of the urethral meatus may reveal stenosis or sequelae of hypospadias. A urethrocutaneous fistula may be detected in some instances, particularly in patients who have undergone previous urethral surgery or have longstanding lower urinary tract obstruction. Palpation of the scrotum, perineum and urethra may reveal thickening or induration of the urethra or associated abscess or epididymitis. Urethral induration often indicates severe spongiofibrosis, but if there is an associated mass effect the diagnosis of urethral carcinoma must be considered. Digital rectal examination may help to evaluate prostatic features in both benign prostatic hyperplasia (BPH) and pelvic fracture urethral injury (PFUI).
Neurological evaluation in the context of a neurogenic bladder or a PFUI is critical because it may affect postoperative care, outcomes such as continence and catheterization, as well as positioning. Severe lower limb spasticity may even contraindicate a urethral reconstruction , favoring upper urinary diversion instead [10].
5.2.3 International Prostate Symptom Score (IPSS) and Other Patient Reported Assessments of Voiding Function
The International Prostate Symptom Score, IPSS (same as AUA-SI, but also including a quality of life domain) is the most widely employed method of patient reported evaluation for bladder outlet obstruction and USx [4, 11]. This questionnaire has been validated in different languages and cultures [12–17]. It was first described for urethroplasty by Morey et al. in 1998 [18] and it is still recommended by the Société Internationale d’Urologie (SIU) and the American Urological Association (AUA) guidelines as part of both preoperative and postoperative evaluation for USx [8, 11]
In the initial assessment of USx, the presence of LUTS, defined as any symptom detected by IPSS, is as high as 92.9% [4]. However, in 2012 Nuss et al. showed that up to 21% of patients presented with symptoms not included on AUA-SI, thus, missing more than one-fifth of the strictures when used alone [11]. Urinary symptoms such as spraying of the urinary stream, post-void dribbling and dysuria are frequently seen in patients with stricture and are not detected by the IPSS. Men with penile USx are more likely to have stream spraying than bulbar strictures were (17% vs. 6%), while LS-associated USx present more frequently with obstructive symptoms (76% vs. 55%) and less frequently with AUR compared with non-LS USx (0% vs. 16%) [11]. Furthermore, bladder stones, prostate and bladder diseases, along with neurourological problems can also modify IPSS scores, causing a low specificity as a major downside for this test [19–21].
Studies reporting the use of IPSS as patient reported measure after stricture treatment were found in 47% of all published articles between 2000 and 2008 [22]. In this scenario, a dramatic decrease in IPSS (improvement) occurs in patients after urethroplasty except in those with an identified recurrence [18]. Additionally, in 2002 Heyns et al. found that IPSS has an inverse correlation with maximum flow rates (Qmax) in patients with USx and thresholds of Qmax < 15 ml/sec and IPSS >10 yielded a sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and overall accuracy (OA) of 93%, 68%, 78%, 89%, and 82%, respectively, for the diagnosis of USx [23].
However, the lack of symptoms does not necessarily mean unobstructed voiding while an unobstructed voiding does not entail the absence of symptoms [22, 24–26]. A systematic review from 2010 showed that there was no robust evidence supporting the use of IPSS as a condition-specific PROM for urethral reconstruction [27]. Likewise, patient satisfaction does not necessarily agree with physician-defined success [28] In 2016, Tam et al. published a multi-centered study evaluating 393 men who underwent anterior urethroplasty [25]. Stricture recurrence was defined as the inability to pass a 17F cystoscope. All patients had IPSS decreased postoperatively, regardless of cystoscopic recurrence and the estimated receiver operating characteristic area under curve (ROC AUC) for IPSS was as low as 0.56, indicating a very poor performance when discriminating between those with or without recurrence. Strikingly, if the IPSS cut point of >10 advocated by Heyns had been used, only 38% of the USx found by cystoscopy as a gold standard (GS) would have been detected [25]. Urethral stricture is clearly more complicated than the simple absence or presence of lower urinary tract symptoms.
On the other hand, sexual dysfunction, chordee, and urethral and bladder pain are significant predictors of dissatisfaction among patients after urethroplasty, emphasizing the need for a comprehensive disease-specific PROM [29, 30]. Currently, there is only one existing PROM for USx assessing LUTS, visual evaluation of the force of the stream, postoperative satisfaction, and quality of life (QoL) along with a visual analogue scale, but unfortunately sexual function is not included in this stricture-specific PROM (USS-PROM) [31–33] (See Appendix 1).
Some of the limitations of the IPSS include poor performance in patients with lower educational level [17, 34, 35]. In order to avoid this concern, a non-validated visual prostate symptom score (VPSS) was developed for assessing LUTS [36]. A study using VPSS was conducted in patients with a previously diagnosed USx found that less educated individuals were more frequently able to respond to the VPSS on their own (68%) than when responding to the IPSS (13%) and VPSS appeared accurate in representing the lower urinary symptoms associated with stricture [37].
More recently, Breyer et al. reported a qualitative study assessing and comparing what patients and urologists considered as their most relevant concerns with regard to USx in the pre- and post-urethroplasty settings. Interestingly, both groups agreed in only 53% of the cases. Patients were mostly worried about “being unable to pee” and having dribbling “in my underwear after peeing”, while physicians considered that straining and weak stream were the most relevant issues [32]. Work is ongoing to further develop a new USS-PROM taking into account more recent findings.
5.2.4 Sexual Function Associated with Urethral Stricture and After Urethroplasty
Coursey et al. were the first to investigate sexual function in men after anterior urethroplasty using a non-validated questionnaire, including circumcised men as a control group. They reported similar incidence of sexual issues after urethroplasty or circumcision [33]. It has been demonstrated that the dorsal nerve of the penis, cavernous nerve, and perineal nerve all interact in providing sensation, motor, and autonomic innervation to the penis and control sexual function and thusly are relevant anatomical landmarks to be considered when performing urethroplasty [38–40]. The impact of USx on sexual health can be divided into three main domains, erectile dysfunction (ED), ejaculatory dysfunction (EjD), and chordee/penile shortening. Probably due to the aforementioned anatomical characteristics, sexual dysfunction (SD) is more commonly present in the context of a pelvic fracture urethral injury (PFUI) than it is with anterior USx [41].
5.2.4.1 Erectile Dysfunction
Preoperative ED, spontaneously reported by patients having anterior USx , without using any questionnaire, has been found in 11–12% as part of their presenting complaint, while prospective studies using the International Index of Erectile Function (IIEF) have found ED in as many as 44% of cases, preoperatively [4, 11, 42].
The IIEF was first used by Anger et al. to prospectively compare the frequency of ED between pre and postoperative setting among 25 subjects undergoing anterior urethral reconstruction [43]. They did not find any significant difference in ED after surgery, however, the incidence of new onset erectile dysfunction was not assessed. Subsequently, ED has been reported in 25–38% at 4 and 2 months after urethroplasty, using the Brief Male Sexual Function Inventory (BMSFI) and IIEF, respectively [44, 45]. Sixty-six percent of individuals had an early postoperative ED, but fortunately, 90% of patients fully recovered at a mean of 190 days postoperatively, which is consistent among studies [42, 46–50].
A meta-analysis from 1997 to 2012 reported de novo ED after anterior urethral reconstruction, with an overall rate of 1% that ranged from 0% to 38% (CI 1–3%) [45]. Only 5 out of 21 studies found a rate ≥20%, while, interestingly, all of these studies used validated questionnaires and were conducted after 2001. Postoperative ED seems to be higher for bulbar (76%) compared with penile strictures (38%) [42]. The authors stated that older studies could have underrated de novo ED, based on the high heterogeneity among studies. They also hypothesized that transient early ED may be attributable to either temporary psychological impact or quickly resolving vascular injuries.
In the setting of PFUI, “it is difficult to differentiate between ED due to PFUI and de novo ED due to urethral realignment or delayed urethroplasty…” [51]. Vascular and neurological damage along with tunica albuginea injury may lead to venous leakage or intracavernosal fibrosis [47]. There is a reported ED rate of 20% in patients after pelvic fracture alone, regardless of the presence of PFUI [52]. Age, severity, type of pelvic trauma, and urethral involvement are associated with a higher risk of ED [48, 49]. A systematic review and meta-analysis of 24 studies, revealed that 34% of patients with PFUI reported ED before undergoing delayed urethroplasty [51]. However, formal urethroplasty resulted in an additional 3% rate of de novo ED [50, 53, 54]. It has even been argued that posterior urethroplasty may improve the erectile function in up to 16–66% of individuals having ED after PFUI [55, 56]. On balance, ED in the setting of PFUI is mainly related to the primary trauma rather than to the reconstruction itself.
5.2.4.2 Ejaculatory Dysfunction
Normal ejaculation depends on several anatomic structures and is under the efferent control of the inferior hypogastric plexus and pudendal nerve rami [38, 57]. Ejaculatory dysfunction may be caused primarily by USx and can potentially occur after urethroplasty. Injury to the bulbospongiosus muscle (BS) may also lead to an impaired ejaculation [58]. In 2012, Rourke et al. found that sexual dysfunction was present in 12.1% of subjects with USx, almost exclusively owing to EjD [4]. More recently, a systematic review showed that validated questionnaires for EjD were utilized infrequently. However, preoperative EjD was present in up to 85% of patients with USx and more frequently in older subjects and patients with bulbar strictures. After anterior urethroplasty, ejaculatory function improves in 12–65%, remains unchanged in 0–23%, and worsens in 0–19% of patients [59, 60]. No significant association was found between stricture length, stricture location or muscle transection and postoperative EjD. However, a higher risk of EjD was associated with advancing age and ventral onlay grafting, possibly related to semen pooling in a urethral pseudodiverticulum [59].
Ejaculatory function data in the setting of posterior urethral stenosis is lacking, predominantly retrospective and exclusively related to PFUI [61, 62]. Preoperatively patients commonly have EjD related to complete urethral obstruction. After reconstruction, 98.3–100% recovered antegrade ejaculation, but 1.7–39.6% had some degree of EjD, which is likely caused by the PFUI rather than the repair itself [61, 62]. Overall, improvements after either anterior or posterior urethroplasty are presumably due to a lower resistance along the urethra after repair.
5.2.4.3 Chordee and Penile Shortening
In 2016, Bertrand et al. prospectively evaluated risk factors for postoperative dissatisfaction after anterior urethroplasty, and found a higher rate of penile curvature (35% vs. 17%) and penile shortening (48% vs. 32%) among dissatisfied individuals [29]. This is consistent with a later prospective study by Maciejewski et al. who found both shortening (OR 2.26, 95% CI 1.38–3.69, p = 0.001) and chordee (OR 2.46, 95% CI 1.44–4.19, p = 0.001) to be independent strong predictors of dissatisfaction after urethroplasty [30]. While chordee and penile shortening are not common after USx treatment their occurrence greatly impacts patient satisfaction and should be captured in clinical assessments.
5.2.5 Uroflowmetry and Post-void Residual Urine Measurement
Uroflowmetry (UFM) is a simple functional non-invasive test which essentially measures the urinary output flow (volume/time) and its characteristics, such as peak and average flow rates (Qmax and Qav, respectively), voiding volume (VV), time, and pattern [63].
The so-called “classic” pattern of USx is a decreased Qmax, flat-shaped curve, and increased voiding time. In spite of this, it has been evidenced that no UFM characteristics can objectively and reliably distinguish between bladder outlet obstruction (BOO) and detrusor impaired contractility [64]. Conditions that may affect UFM, other than USx, are impaired detrusor contractility, BPH, detrusor sphincter dyssynergia, and pelvic floor dysfunction. The simultaneous measurement of both UFM and detrusor pressure (Pdet), namely, the pressure-flow study, is the only way to reliably confirm obstruction [64–66]. Despite these limitations UFM is commonly used to evaluate USx.
With respect to the initial diagnosis of USx, Heyns et al. demonstrated that Qmax had a significant association with both IPSS (negatively) and urethral caliber (positively) among patients presenting to a urology clinic with USx [23]. When combining the cutoff values of Qmax < 15 ml/sec and IPSS >10, they obtained a 93% sensitivity, 68% specificity, and 82% OA, while only 4.3% of USx would have been missed. On the other hand, different studies reported preoperative mean Qmax ranging from 9.4–11.8 ml/sec [24, 60]. More recently, Lambert et al. designed a mathematical model to discriminate between USx and BPH based in UFM parameters only. The model achieved a sensitivity of 80%, specificity of 78%, PPV of 76.9%, NPV of 79.2%, and a ROC AUC of 0.84 when discriminating between BOO secondary to BPH vs. USx [67]. However, the model could not distinguish decreased flow due to BOO from impaired detrusor function and the authors advise against its use in diabetics or subjects with possible neurological conditions. At any rate, both AUA and SIU guidelines on USx recommend the use of UFM in the preoperative evaluation of USx [8, 9, 68].
In the post-urethroplasty context, UFM has been used in 56% of published papers [22]. In these circumstances, the main question is whether UFM can replace invasive testing when detecting stricture recurrence after urethroplasty or not. Uroflowmetry diagnostic performance typically depends on what test is being used as a GS and on stricture prevalence in the reference population. Because of these factors there has been generally inconsistent results. When a Qmax cutoff <10 ml/sec is used alone, Erickson et al. reported a 54% sensitivity, 93% specificity, 73% PPV, and 86% NPV in detecting failures after repair, when retrograde/voiding urethrogram (RUG/VCUG) were utilized as a GS. Interestingly, they showed that 30% of recurrences had a Qmax > 15 ml/sec inferring that 30% of recurrences would be missed with the use of UFM alone. Conversely, by means of a subjective interpretation of UFM curve, they found 93% sensitivity and 84% specificity [69]. Later, it was demonstrated that ∆Qmax (postoperative Qmax − preoperative Qmax) was a much better predictor of failure than Qmax alone [60].
In 2016, Tam et al. published a prospective multi-institutional study including 323 subjects who were followed-up over a mean of 12.8 months after anterior urethroplasty [24]. Unlike the previous studies, they used cystoscopy as a GS. There was no difference when comparing either postoperative Qmax or a novel Qmax-Qa value. For a Qmax cutpoint of <10 ml/sec, they found a sensitivity as low as 21%, while for a Qmax-Qa of ≤10 ml/sec sensitivity was 83% and specificity only 58%. Both Qmax and Qmax-Qa had a significantly better performance for patients ≤40 years, likely due to a higher prevalence of BPH and weak detrusor among the elderly [24]. Therefore, the use of UFM alone as a screening for USx recurrence seems to have an unacceptably poor performance. Despite the above, using preoperative UFM along with patient reported measures is consistently recommended as a baseline to compare future outcomes, incorporating patient’s perspective [8, 68]. Likewise, several UFM calculated values along with PROMs may give an acceptable screening yield for recurrences, but only if cystoscopy is performed at some point of the follow-up [18, 23, 25, 37, 70]. On balance, uroflowmetry may be a useful “screening” tool to diagnose USx but confirmation of a USx requires either cystoscopy or urethrography.
Post-void residual (PVR) urine , measured by either in-and-out catheterization or ultrasound, is considered part of the objective assessment of the voiding function alongside UFM, and has been reported as a screening test in 8% of studies [22]. However, many factors can alter PVR urine findings and an elevated PVR urine likely only occurs in the setting of detrusor decompensation, so it should likely never be used alone for primary diagnosis of USx or for follow-up after stricture surgery [70]. In any case, PVR may be valuable as an adjunctive test in determining the risk of bladder failure or impending upper tract dysfunction secondary to chronic urinary retention due to USx [70].
5.3 Invasive Tests: Definite Diagnosis and Staging
5.3.1 Cystourethroscopy
Direct visualization of the urethra and the bladder has been present in urological practice as a diagnostic tool for a very long time [3]. Either rigid or flexible cystoscopes can be found in almost every urology service and almost every urologist at every level of training is able to use them properly. Cystourethroscopy (CU) has a critical importance for the reconstructive urologist because “as a diagnostic modality, cystoscopy has been considered the gold standard for determining the presence or absence of a urethral stricture” [68].
It has been claimed that RUG/VCUG alone may be sufficient to definitively diagnose and stage a USx. Nonetheless, not to perform CU may lead to missed valuable information, such as the presence of concurrent lower urinary tract pathology such as tumor, urinary calculi, hair-bearing tissues (after prior urethroplasty), bladder neck status, BPH or simply unhealthy urothelium.
While the AUA guidelines declare that either RUG/VCUG or CU are necessary to establish a USx diagnosis, the SIU guidelines state that CU and RUG are recommended as Grade A investigations, VCUG at Grade B, and urethral ultrasound at Grade C. On one hand, RUG/VCUG are able to accurately estimate the length and location of the stricture, while on the other hand as a binary measure, CU is the most specific test for diagnosing USx but is not able to accurately determine stricture length [8, 9, 68, 71, 72].
In 1968, Smith demonstrated that the urinary flow decreases and symptoms occur once the urethral caliber is <11F. Before that, the bladder can maintain a normal urinary flow at the expense of abnormally high voiding pressures, therefore, symptoms which make the patient seek medical care may become evident long after urinary pressures have been present for a long period of time [73]. Thus, complications may arise from undiagnosed USx or urethroplasty failures in the absence of decreased flow or urinary symptoms [4, 6]. In this respect, several prospective studies have consistently concluded that the best way to define anatomical success and to compare outcomes between institutions after urethral repairs is “the ability to pass a 16F-17F cystoscope beyond the area of reconstruction with ease”, overcoming the performance of UFM, PROMs, and even that of RUG/VCUG [26, 74, 75]. But this may be far from real practice, in 2013, Yeung et al. published a survey of members of the Society of Genitourinary Reconstructive Surgeons in which 60% of respondents defined surgical failure as the need for a secondary procedure, 14.4% did so based on RUG/VCUG findings, and only 12.2% used CU [76].
Our recommendation is that once BOO is suspected, a physical examination alongside with non-invasive tests, such as questionnaires and UFM and PVR urine measurement, may be utilized as a screening assessment. However, if USx is a likely diagnosis, a CU should be booked before or simultaneously with a RUG/VCUG. Preferably, CU is performed under local anesthetic using a 16F-17F flexible instrument. In certain cases, such as meatal/fossa navicularis strictures, we have found that a semi-rigid pediatric cystoscope (6F-8F) can be of great help and provide valuable information about stricture length, urethral caliber, presence of LS, and severity of spongiofibrosis with minimal urethral trauma [75]. Ideally, CU should be performed with the patient lying on a radiolucent table, so that, a RUG/VCUG can be done simultaneously.
Cystourethroscopy does have some potential disadvantages. First of all, it is an invasive test, thus, individuals may complain of postprocedural hematuria, bladder/urethral pain, and rarely urinary tract infections. Secondly, it can be costly in certain environments [77]. Thirdly, after urethroplasty up to 35% of subjects with a cystoscopic recurrence were asymptomatic. Thus, it is unclear if cystoscopy is an overly sensitive means of detecting USx. These factors may limit the use of CU during follow-up, especially when patients have minimal symptoms (normal flow, no LUTS, normal EjF, etc.) and it has been reported that only 54.4% of patients are compliant with a 1-year cystoscopic follow-up after urethroplasty [26]. One final important consideration is that the vast majority stricture recurrences can be diagnosed within the first year with cystoscopic assessment and cystoscopy is likely not required long-term after intervention [78]. If cystoscopy was used indefinitely to detect long-term stricture recurrence after urethroplasty, approximately 110–162 normal CU would be required to detect 1 recurrence on a yearly basis [79, 80]. It seems clear that cystoscopy is not required long-term but is likely required at least once after urethroplasty as a means stratify the risk of recurrence. At risk individuals can then be followed more closely in order to adapt to different clinical scenarios and care models.
5.3.2 Retrograde Urethrogram and Voiding Cystourethrogram
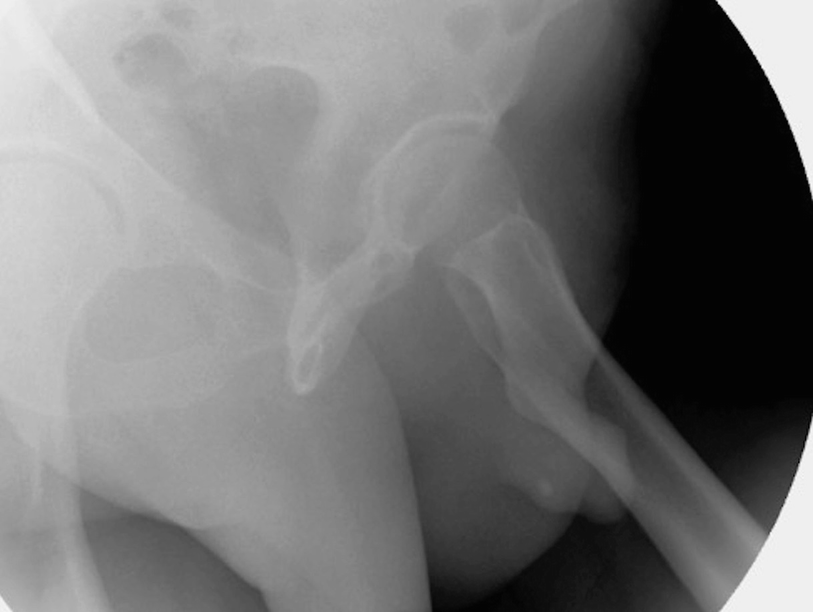
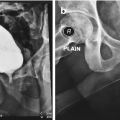
Proper positioning for retrograde urethrogram. In particular, note the occlusion of the downward left obturator fossa. Also, an incidental urethral stone is also evident
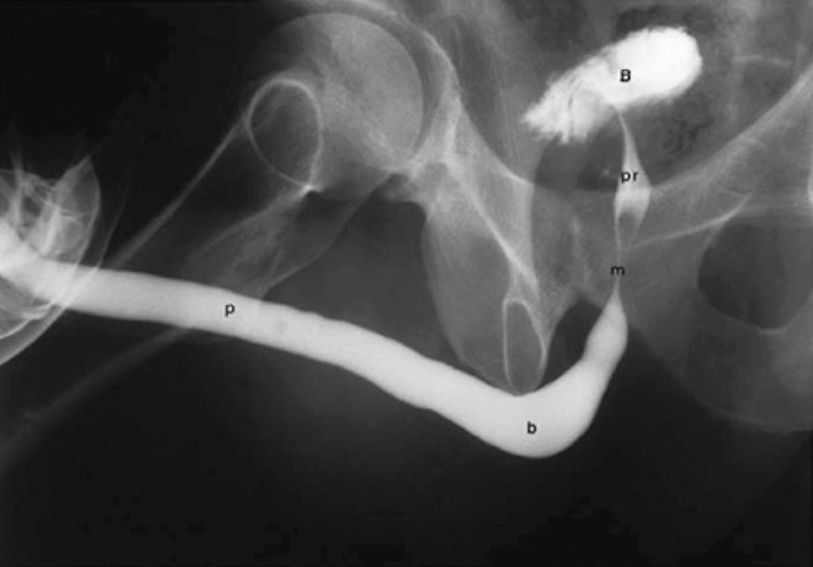
A properly performed and normal retrograde urethrogram . The four distinct parts and bladder are labelled accordingly: p = penile urethra, b = bulbar urethra, m = membranous urethra, pr = prostatic urethra, b = bladder
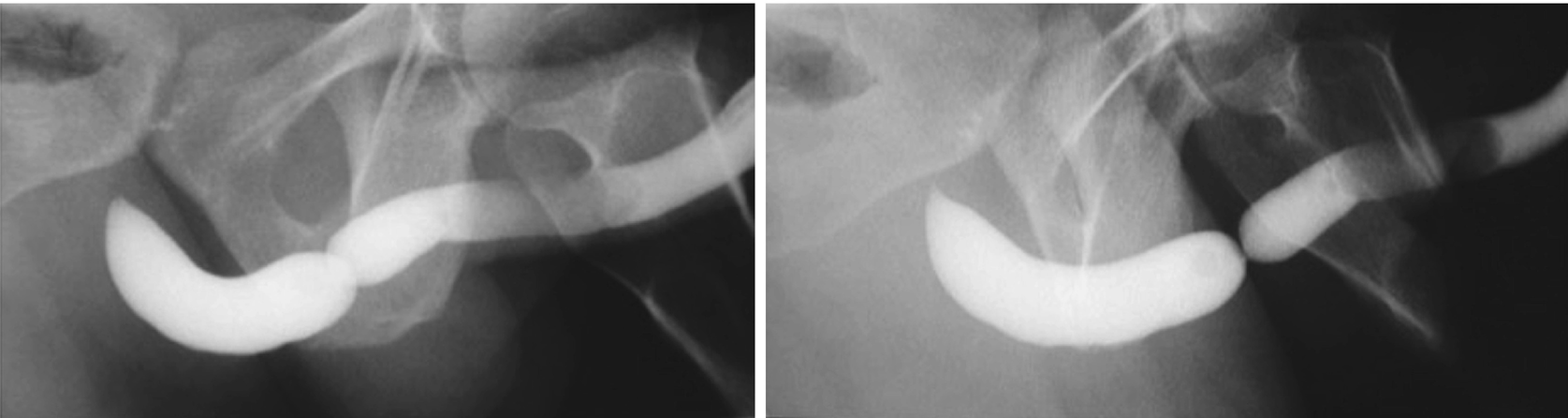
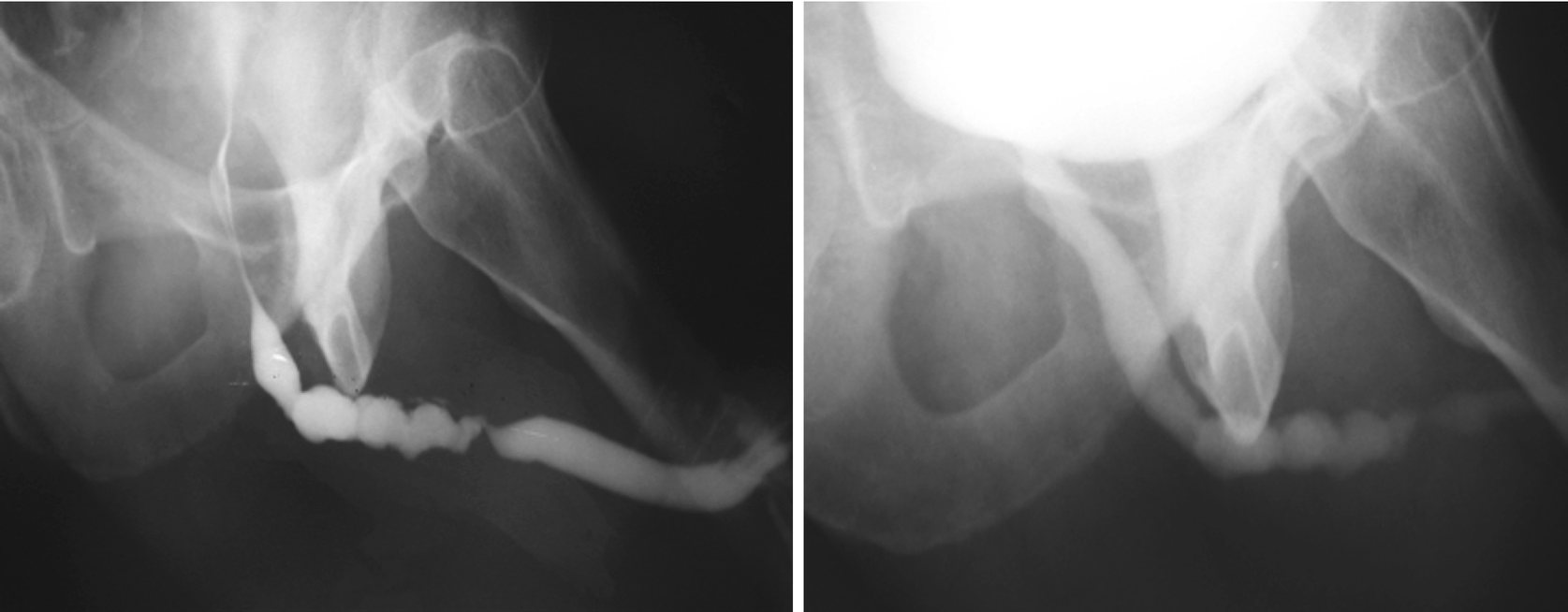
These two urethrograms on the same patient without and with proper oblique positioning demonstrate the importance of proper positioning during urethrogram. Without a sufficient oblique positioning the presence and length of stricture can be underestimated
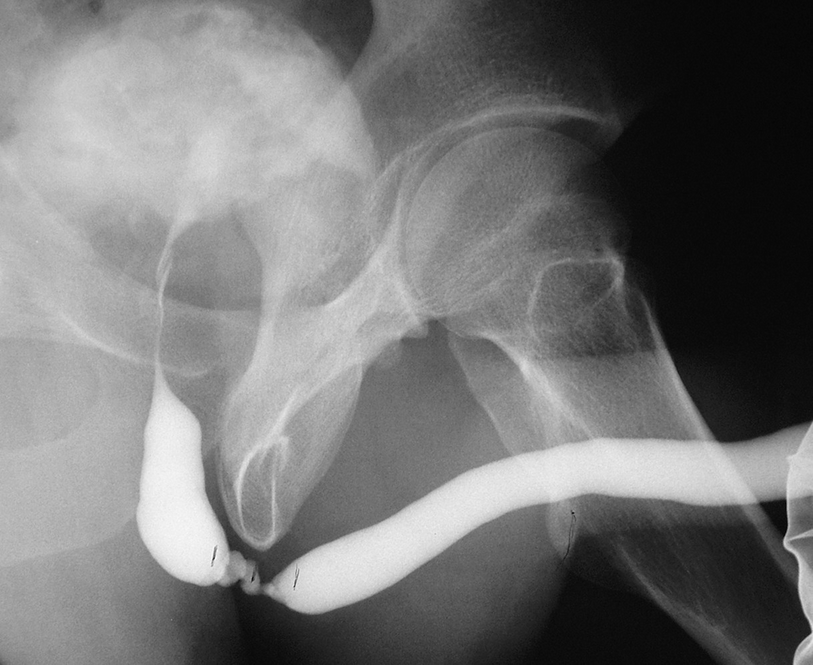
A short segment bulbar urethral stricture identified on retrograde urethrogram
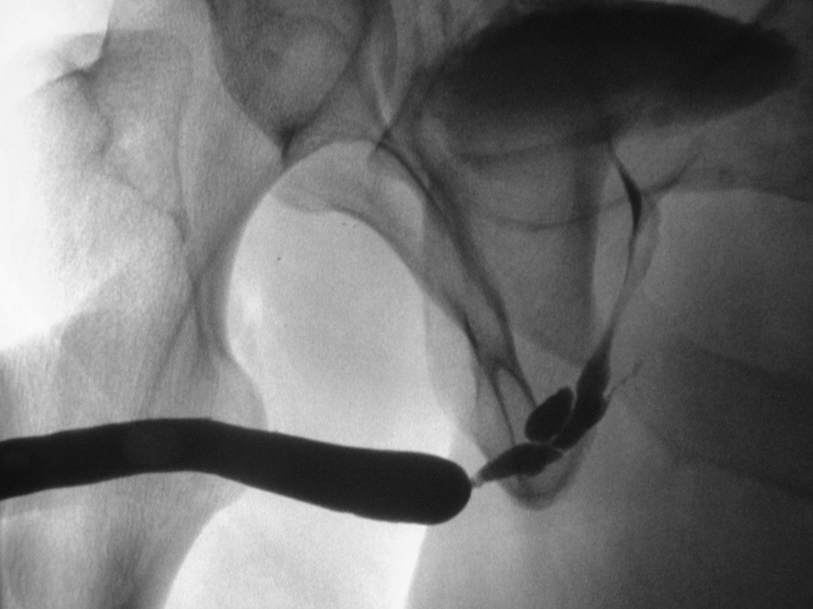
A longer and more complex bulbar urethral stricture with false passages. Note the concurrent filling of Cowper’s gland
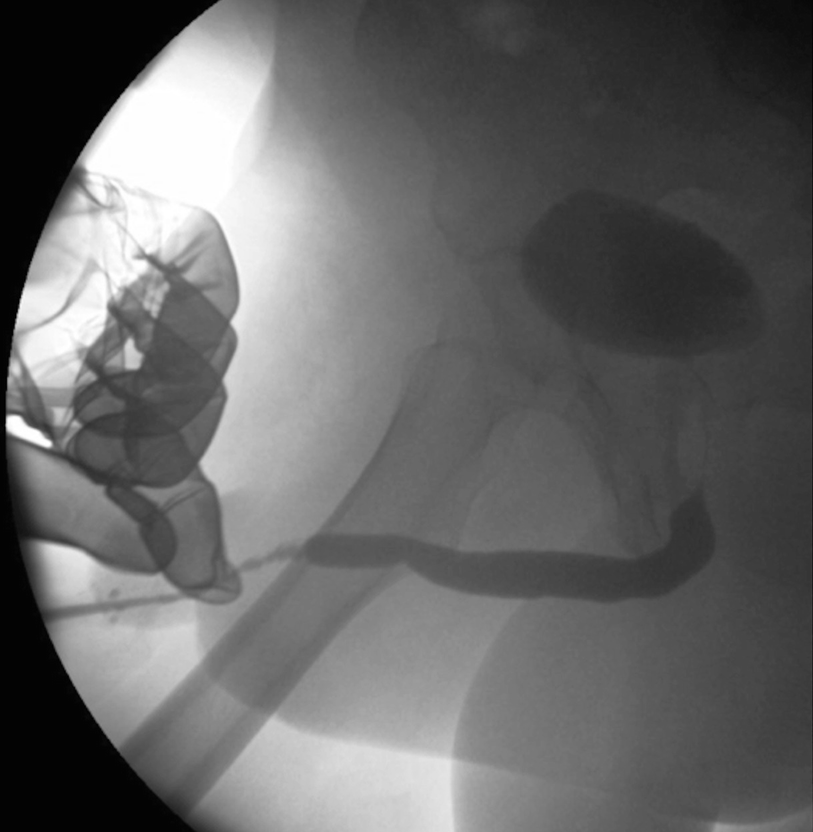
A retrograde urethrogram demonstrating a penile urethral stricture related to prior hypospadias surgery
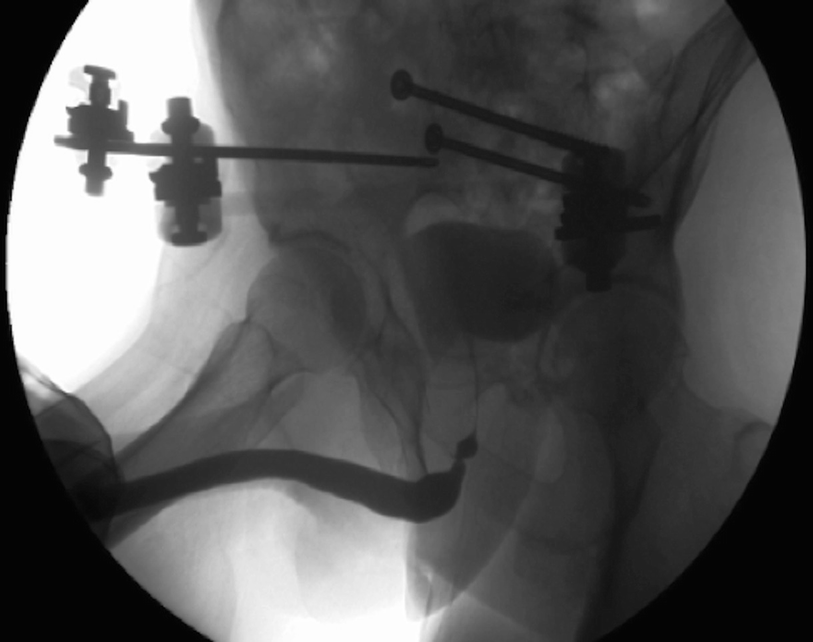
A retrograde urethrogram demonstrating a non-obliterated urethral stenosis associated with a pelvic fracture injury

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree