Poxviruses
|
Poxviruses are double-stranded DNA viruses that replicate in the cytoplasm of host cells (Table 195-1). These “brick-shaped” viruses constitute the largest known animal viruses and can be seen with light microscopy.1 The poxviruses that cause significant disease in humans are reviewed here. Their effects on the host range from systemic disease to localized infection to epithelial cell proliferation alone.
Genus | Species | Hosts | Main Portal of Entry | Clinical Features |
|---|---|---|---|---|
Orthopoxvirus | Variola virus | Humans | Respiratory tract | High fever and myalgias precede oropharyngeal enanthem and centrifugal exanthem. Simultaneous progression of skin lesions from macules to papulovesicles, pustules, and crusts, resulting in significant scars. Last reported case in 1978; concern for use in bioterrorism. |
Vaccinia virus | Humans | Skin | Used as vaccine for smallpox and monkeypox. Vaccination site progresses from papule to vesicle, pustule, and crust, leaving scar. Adverse events occur when virus spreads locally or in a generalized manner, more severe in individuals with disruption of the skin barrier or immunocompromise. | |
Monkeypox virus | Rodents, humans, monkeys, anteaters | Skin | Clinical presentation similar to that of smallpox but more prominent lymphadenopathy and lower mortality. | |
Cowpox virus | Rodents, cats, humans, cattle | Skin | Contact with infected animal host gives rise to papule that becomes vesicular, hemorrhagic, pustular, and ulcerative; resulting eschar heals over 3–4 weeks with scarring. Pustular stage: often umbilicated with surrounding zone of erythema and edema. Constitutional symptoms and lymphangitis common. Can be extensive or severe if skin barrier disrupted. | |
Parapoxvirus | Orf virus | Sheep, goats, humans | Skin | Contact with infected animal or fomite leads to papule(s) that forms pustules or nodules with central umbilication and surrounding gray-white or violaceous ring and outer zone of erythema; lesion usually occurs on hand. Lesions become weepy then dry and crust. Healing occurs over 4–8 weeks, usually without scarring. Constitutional symptoms/lymphangitis occur less commonly. |
Paravaccinia virus | Cattle, humans | Skin | Transmission by contact with infected teats/mouths of cattle. Clinical findings and course similar to those of orf; differentiated by their different animal hosts. | |
Bovine papular stomatitis virus | Cattle, humans | Skin | Transmitted by contact with infected mouths of cattle. Clinical findings and course similar to those of orf and paravaccinia. | |
Molluscipoxvirus | Molluscum contagiosum virus | Humans, chimpanzees (one report) | Skin | Discrete firm, dome-shaped papules; central umbilication. Can be extensive in individuals with atopic dermatitis or immunocompromise. Most cases self-resolve in 9–12 months but lesions often treated to speed resolution; can be persistent/refractory in immunocompromised individuals. |
Yatapoxvirus | Tanapox virus | Humans, monkeys | Possibly skin | Uncertain mode of transmission, possible mosquito vector from infected monkeys to humans. Short fever precedes eruption of one to multiple pruritic, indurated papules with surrounding edema. Become necrotic and/or ulcerative then heal within 6 weeks with scarring. |
Orthopoxvirus Infections
|
Variola virus is the pathogen responsible for smallpox, a disease that devastated humankind in catastrophic epidemics for over 3,000 years. This poxvirus scarred and killed millions of people in both the Old and New Worlds, affecting entire populations on every continent.
Edward Jenner altered the course of history in 1796 when he created the first vaccine by inoculating patients with cowpox virus in order to protect patients against the related smallpox virus. Utilizing this method of protection, smallpox could be prevented, and an intensive global effort at vaccination and tracking of cases led to its eradication in 1980.2 It subsequently appeared an illness of merely historical interest until threats of bioterrorism in recent years renewed the need for knowledge about smallpox and its features, along with the development of improved vaccines and treatments for use should the disease arise again.
Unlike other diseases caused by members of the family Poxviridae, smallpox affects only humans and thus cannot be acquired from another species. Transmission is generally via respiratory droplets and requires close contact. Outbreaks occur in the winter and early spring seasons when conditions of low humidity and low temperature favor survival of the aerosolized virus.3 Smallpox is less infectious than other diseases spread by the respiratory route, including measles, varicella, and influenza. Secondary attack rates for unvaccinated contacts are estimated to range from 37% to 88%.4 Secondary cases are often limited to family members or health care workers. Spread of smallpox is enhanced when there are large quantities of virus in the aerosolized droplets, an increased number and extent of exposures between infected individuals and contacts, and a higher population density. The very young, elderly, and pregnant women are more susceptible to infection.5 Individuals with more severe clinical disease are reported to be more infectious, but these same people also tend to be toxemic and confined to bed.
Because no significant subclinical carrier state exists, eradication was possible and was accomplished in 1980. After its eradication, the responsible virus was presumed to be limited to two World Health Organization (WHO)-approved laboratories in the United States and the former Soviet Union (Russian Federation). A theoretical concern exists that these or other unreported stocks of virus may be in the hands of countries or groups with terrorist relationships and could be aerosolized for use as a biologic weapon (see Chapter 213). The possibility of genetically altering variola or other similar viruses for increased virulence further heightens concerns regarding misuse.6 The majority of the population would be susceptible to smallpox disease as routine vaccination of civilians was discontinued in the United States in 1972 and worldwide in the 1980s. Those vaccinated before 1972 have uncertain levels of immunity remaining. Until 2003, laboratory workers handling nonhighly attenuated orthopoxviruses were the only individuals recommended to receive routine vaccination. Since that time, military personnel and a small group of US civilians who would serve as first-line responders in a possible outbreak have been vaccinated.7 The risk of untoward effects from the vaccine, particularly in immunocompromised patients and individuals with atopic dermatitis or other diseases where a skin barrier defect exists, caused health authorities to hesitate before implementing mass vaccination policies.
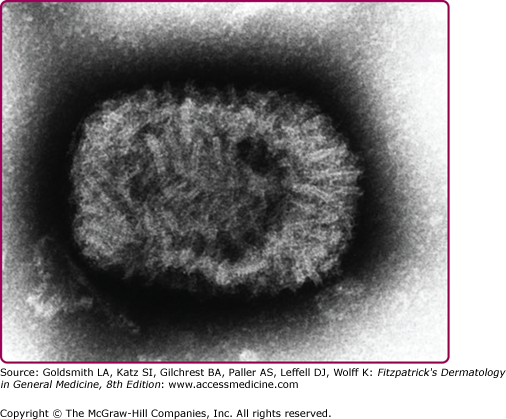
Smallpox is caused by the variola virus, a linear, double-stranded DNA virus of the genus Orthopoxvirus. Variola virus measures approximately 300 × 250 × 200 nm and has an oval- or brick-shaped appearance on electron microscopy (see eFig. 195-0.1). Strains of the virus fall into two main groups: (1) variola major and (2) variola minor. Although their genomes share approximately 98% homology, their virulence is markedly different, with 30% or higher mortality associated with the major type and less than 1% mortality associated with the minor type.2,8
Humans are the only natural reservoir of the variola virus and the reason for this strict tropism is not fully understood.9 The disease process begins following close, prolonged exposure to an infected individual. The usual portal of entry is the oropharyngeal or respiratory tract, either by inhalation of aerosolized droplets or direct contact with infected mucous membranes.10 After entry, the virus attaches to respiratory epithelial cells, travels to regional lymph nodes, and replicates at these sites. Transient primary viremia with uptake of the virus by macrophages occurs, and the variola virus spreads to the reticuloendothelial organs, where asymptomatic replication continues. A massive secondary viremia follows and causes the onset of symptoms (the prodromal period). The virus spreads to the skin and mucosa, along with other organs and tissues such as the liver and kidneys.11 Variola virus is transmissible beginning in the late prodromal phase through aerosolization of viral particles from the oropharynx. It is most infectious during the first 7–10 days after onset of the viral rash and remains transmissible until the scabs fall off of skin lesions.11,12
Variola virus is less commonly spread via accidental inoculation into the skin, through the conjunctiva, and through contact with infected body fluids or highly contaminated fomites. Rarely, it has been transmitted transplacentally and by long-range airborne or suspended viral particles in enclosed areas.6
The infected individual remains asymptomatic during the incubation period of viral replication. A prodrome of high fever [39°C–41°C (102.2°F–105.8°F)], chills, myalgias, and severe headache develops within 7–17 days of exposure, with an average incubation of 12 days for variola major and a few days longer for variola minor. The person is usually severely ill and bedridden during the prodromal period, which lasts 2–4 days. Children may develop seizures.4,10
Evanescent, nonspecific urticarial or morbilliform lesions can develop during the prodrome. These occur mainly in previously vaccinated individuals at the vaccination site and the axillae, popliteal, and groin areas.2 Approximately 1 day after the onset of fever, an enanthem develops. The earliest viral lesions are red macules on the mouth, tongue, and oropharynx that subsequently vesiculate and ulcerate, releasing high concentrations of transmissible virus particles in respiratory secretions. A skin rash (exanthem) erupts two to several days after the onset of fever, at about the same time that the mucosal lesions ulcerate. The fever usually declines with appearance of the rash.6,13 The exanthem varies depending on viral dose and strain, vaccination status, level of immunity, and nutritional status.5 The outbreak is categorized into two clinical forms: (1) variola major and (2) variola minor.
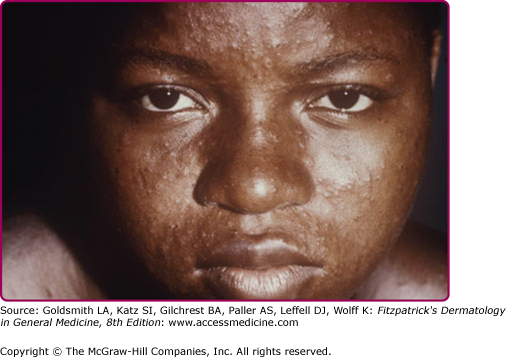
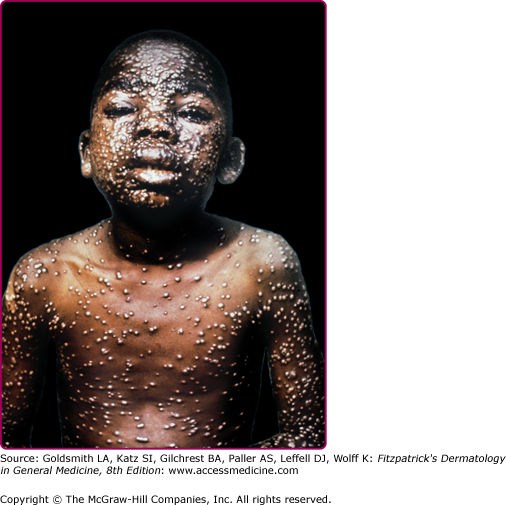
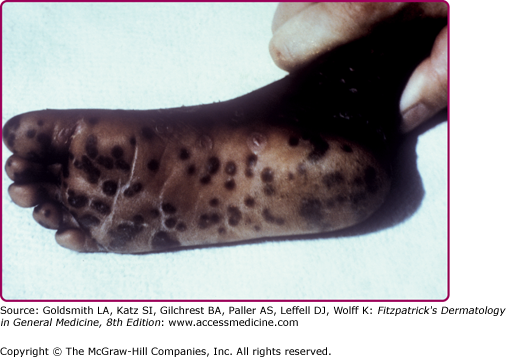
Forms of variola major are more common than variola minor. One classification divides variola major into five clinical types.14 Ordinary, common or classic smallpox is the most common type and accounts for over 90% of cases of smallpox in unvaccinated individuals. Skin lesions begin as macules on the face and upper extremities that then spread quickly to the trunk and lower extremities. Involvement of all parts of the body, including palms and soles, occurs within 24–48 hours. The macules become raised papules within 1 day and then form vesicles, often with central umbilication, after 4–5 days (see eFig. 195-0.2). Fever recurs as the lesions become firm pustules around day 7 (Fig. 195-1) and persists until all lesions have crusted and scabbed at day 14 (Fig. 195-2). Scabs separate by 3–4 weeks after the onset of the rash. Key features of smallpox skin lesions are their prominence peripherally on the face and extremities and their simultaneous progression in a centrifugal pattern, with all lesions in any one area exhibiting the same morphology.10,15 After their resolution, pitted scars (“pockmarks”) often persist and can be disfiguring (Fig. 195-3). Scarring is most common on the face, where there are larger and more numerous sebaceous glands, which are particularly susceptible to infection and destruction by variola virus.2
Modified smallpox has as severe a prodromal illness as ordinary smallpox, but the clinical course of cutaneous lesions is accelerated. Lesions are fewer in number and tend not to progress to vesicles or pustules, crusting by day 10. This form occurs mainly in vaccinated individuals and does not cause death. Flat smallpox (also called malignant smallpox) is an uncommon form of variola major in which the macules barely raise. It usually occurs in children and unvaccinated individuals lacking cellular immunity. Those affected become severely ill with toxic fever, and most die with hemorrhagic lesions and pneumonia.4 The hemorrhagic, or fulminant, type of variola major is equally common in unvaccinated and vaccinated persons. It is divided into two forms: early and late. The early form consists of petechial hemorrhages into the skin or mucous membranes during the prodromal period. Massive hemorrhage from mucosal surfaces leads to death within 8 days of onset, before any appearance of the typical rash of smallpox, with a case fatality of 100%. Women, especially if pregnant, are more susceptible to this form. In the late form, hemorrhage appears after onset of the typical rash, death occurs by 12 days after onset, and men and women are equally affected.16 The last type of variola major is variola sine eruptione, in which vaccinated individuals come in contact with affected individuals and develop fever and other symptoms (e.g., headache, conjunctivitis) but no rash. In this type, the illness lasts 48 hours or less. Serologic studies do show a rise in antibody titers against smallpox virus.4,14
Variola minor is clinically indistinguishable from cases of modified smallpox and from cases of ordinary smallpox in which lesions are discrete rather than confluent. Occasionally, the lesions are nonumbilicated. In the pre-eradication period, the diagnosis of variola minor was given only after assessment of the severity of an outbreak, if the case fatality rate was low (1% or less).8 Molecular virologic differentiation between major and minor viral strains is now available.
Variola virus spreads via the blood to affect other noncutaneous systems. It can infect the metaphyses of growing bones and lead to arthritis in up to 2% of affected children. Osteomyelitis variolosa occurs less frequently than arthritis, but may also cause bone deformities.17 Swelling of the eyelids and a mild conjunctivitis are common findings. Cough and bronchitis may be seen in some cases of smallpox. A degree of encephalopathy often occurs, with symptoms ranging from headache and hallucinations to delirium and psychosis.2 Gross hematuria can occur with the hemorrhagic type of variola major.16
The white blood cell count may increase as the skin lesions of smallpox become pustular. Severe thrombocytopenia occurs in both early and late hemorrhagic smallpox. A marked decrease in the level of factor V (accelerator globulin) and increase in thrombin time are noted in the early form, likely from disseminated intravascular coagulation. The late form has a smaller degree of these coagulation disturbances.2
Skin biopsy specimens from early papules show edema and dilation of the capillaries of the papillary dermis with a perivascular infiltrate of lymphocytes, histiocytes, and plasma cells. With progression, the cells of the epidermis become vacuolated and swollen and undergo ballooning degeneration. These vesicles have characteristic intracytoplasmic inclusion bodies called Guarnieri bodies. Pustules form with migration of polymorphonuclear cells into the vesicles. Eventually, the pustule becomes a crust, with new epithelium growing to repair the surface. Mucous membrane lesions show similar changes but also have extensive necrosis of the epithelial cells leading to rapid ulceration rather than vesiculation.18
For diagnosis, specimens (skin lesions, tonsillar swab, blood, skin biopsy) should be collected by someone recently vaccinated and sent to designated high-containment facilities.
Scrapings of skin lesions can be examined via electron microscopy to assess for the typical oval or brick shape of orthopoxviruses. Serologic testing for orthopoxviruses with paired samples can be performed; this identifies the presence of a member of the genus but is not specific for variola virus. Polymerase chain reaction (PCR) methods are used to definitively identify variola virus and can also characterize the viral strain. Variola virus can be cultured in several commonly used cell lines and can be identified by the formation of characteristic pocks on chorioallantoic membranes of chicken embryos.6,19
(Box 195-1). The exanthem of smallpox is most often confused with that of varicella (chickenpox). Smallpox lesions are initially centrifugal with simultaneous progression of all lesions. In contrast, varicella lesions have a more truncal (centripetal) distribution, the vesicles are more superficial, and lesions are present at different stages. Varicella also has a shorter disease course, with only a 1–2-day prodrome and with all lesions crusting in 4–6 days from their initial appearance.11 Human monkeypox clinically resembles smallpox but often manifests lymphadenopathy. It is a zoonotic disease and is not spread as easily between persons. The morbilliform prodromal rash of smallpox can be confused with measles or coxsackievirus infections. Secondary syphilis should be considered, especially when there are lesions on the palms and soles, but these lesions do not progress. Other eruptions in the differential diagnosis are listed in Box 195-1. The lesions of hemorrhagic smallpox can be similar to those of meningococcemia, viral hemorrhagic fevers such as Ebola, dengue or rift valley fever, severe acute leukemia, and other acute hemorrhagic eruptions such as those associated with coagulopathies.
Most Likely
Consider
Always Rule Out
|
Secondary bacterial infection occurs commonly in skin lesions as well as at regional lymph nodes, affecting 5% of individuals. A temperature spike occurring 3–5 days after the start of the prodrome may indicate secondary infection.13 Keratitis and corneal ulceration, common in malnourished individuals, result in blindness in 1% of cases. Either variola virus or bacterial superinfection can lead to respiratory complications, including pneumonia, at day 8–10 of illness. Both arthritis and osteomyelitis can lead to limb deformities, including bone shortening, subluxation, and flail joints. Orchitis is less common and usually unilateral. Encephalitis is reported in 0.2% of cases.2
The most common form of variola major, common smallpox, is associated with a mortality rate ranging from less than 10% when lesions are discrete to 50%–75% when lesions are confluent. Death often occurs between days 10 and 16 of illness. Modified smallpox is associated with less than 10% mortality. In contrast, flat smallpox has a case fatality rate of over 90%, and hemorrhagic forms have nearly 100% mortality. The overall mortality rate for variola major is 30%, compared to less than 1% for variola minor.14 Those who survive either disease have lifetime immunity. Death from smallpox is thought to be secondary to toxemia associated with immune complexes and variola antigens, which induce hypotension/shock and multiorgan failure. Encephalitis is an important factor in death from variola minor but not from variola major.2,4
There is currently no specific treatment for smallpox. A patient suspected of having the disease should be isolated in a negative-pressure room and given supportive care. Precautions should be taken to prevent secondary infection and appropriate antibiotics administered should it occur. Research on antiviral agents is ongoing. Intravenous cidofovir, a nucleotide analog approved for treatment of cytomegalovirus infection, is available from the Centers for Disease Control and Prevention (CDC, Atlanta, GA, USA) through an Investigational New Drug protocol. It has been found to have activity against variola, vaccinia, and other orthopoxviruses in in vitro and animal studies, but lacks clinical trial data and does carry a risk of nephrotoxicity.20 Lipid-soluble cidofovir-derivatives, such as CMX001, are under study.21 In addition, the oral drug ST-246 (Tecovirimat, SIGA Technologies, Corvallis, OR, USA) appears to inhibit the function of a major envelope protein (p37) required for extracellular egress of orthopoxviruses and prevent viral spread. It has been used by Emergency Use Authorization since 2008, and has shown efficacy against variola, monkeypox, and other orthopoxviruses in animal studies, and is now in Phase II trials.22 Topical idoxuridine may be used to treat corneal lesions, although its efficacy has not been proven.
Because of the development of lifelong immunity after recovery from natural smallpox infection, the first efforts at prevention were to introduce crusts or fluid from lesions to unaffected individuals to induce mild disease. This process of variolation did reduce morbidity and mortality but also caused full infection and spread in some cases. In 1796, Dr. Edward Jenner developed vaccination, using the cowpox virus to introduce cross-immunity against the variola virus. Vaccinia virus later became the virus used. Vaccination is 90%–96% effective in preventing smallpox disease when given before exposure to variola virus. For postexposure prophylaxis, smallpox vaccination within 2–3 days of exposure can protect against severe disease. Vaccination within 4–5 days may protect against death.2,23 However, vaccination does not give lifelong immunity. The duration and degree of protection over time are subjects of debate. Most estimates suggest that primary vaccination gives full protection for 3–5 years and some but declining immunity at 10 years and after. Revaccination may give significant protection for at least 30 years.12,24
The vaccinia virus is also of the genus Orthopoxvirus. Its origins are unknown, but it is most similar to the cowpox virus in this family. In the nineteenth century, it replaced cowpox as the agent used for smallpox vaccination. Discussion here focuses on its features as the smallpox vaccine, as vaccinia virus is not known to cause natural infection. Because of significant homology with other poxviruses, vaccination with vaccinia virus provides not only protection against smallpox, but also against closely related orthopoxviruses such as monkeypox, camelpox, and cowpox.2
Eradication of smallpox was made possible by intensive tracking of cases of infection with ring vaccination of primary and secondary contacts. The last known case of natural infection was one of variola minor in Somalia in 1977. One final case of accidental laboratory infection occurred in 1978, and the WHO declared eradication in 1980. Routine vaccination of civilians was discontinued in the United States in 1972 and worldwide in the 1980s. Vaccination of US military personnel initially ceased in 1990 and laboratory workers handling nonhighly attenuated orthopoxviruses were the only group still recommended to receive periodic vaccination.25 But with the postal anthrax and World Trade Center attacks in 2001 and other threats of bioterrorism, vaccination was reinitiated in the United States in late 2002 for the military and a small group of voluntary public health and health care workers who would be first-line responders in a possible outbreak.26
Vaccination made eradication of smallpox possible, but given that vaccinia is a live virus, it does carry its own set of adverse effects. Most adverse events can occur at any age, but infants and children younger than the age of 5 years tend to be particularly affected. Adverse reactions are ten times more common with primary vaccination than revaccination. Individuals with atopic dermatitis, or other diseases with skin barrier dysfunction can acquire a severe eruption, eczema vaccinatum, from secondary contact more often than from direct vaccination.4,26
Smallpox vaccination involves the introduction of vaccinia virus into the outer layers of the intact skin. There are many strains of the virus, with the less pathogenic New York City Board of Health (NYCBH) and Lister-Elstree strains being used during the global smallpox eradication campaign. The previous vaccine licensed and used in the United States was a lyophilized, calf-lymph vaccine (Dryvax, Wyeth Laboratories, Marietta, PA, USA) produced from the NYCBH vaccinia strain.12 In 2007, the United States transitioned to the new ACAM2000 vaccine (Acambis, Cambridge, MA, USA), formulated using a clone of Dryvax and modern cell-culture technology.27 ACAM2000 was developed for a number of reasons: the possibility of contamination of the Dryvax vaccine with bovine spongiform encephalopathy from calves, expiration of available Dryvax vaccine, rising concerns of the use of variola for bioterrorism and having an adequate vaccine supply, and risk of hypersensitivity reactions with Dryvax.27 ACAM2000 has thus far shown comparable safety and efficacy to its predecessor in clinical trials and is currently used by the US Department of Defense for military and laboratory worker immunizations.28–30
After injection of vaccine into the skin by a bifurcated needle (see eFig. 195-3.1), the virus rapidly multiplies locally and occasionally at regional lymph nodes. Viable, transmissible vaccinia virus is present at the resulting skin lesion until the lesion scabs and separates. The area heals with scarring at the injection site. Infection is usually limited by the host response with the development of antibody- and cell-mediated immunity.11 Adverse events and complications occur when the virus spreads outside of the local area, either by transfer or a host inability to contain the response.
Soreness is almost universal at the vaccination site. Systemic symptoms can occur and are considered normal reactions. These include fever [greater than 37.7°C (99.9°F) and more common in children], chills, headache, myalgias, and malaise. They generally peak at days 8–10 and last 1–3 days. Approximately 30% of vaccinees feel too ill to carry out normal activities.31
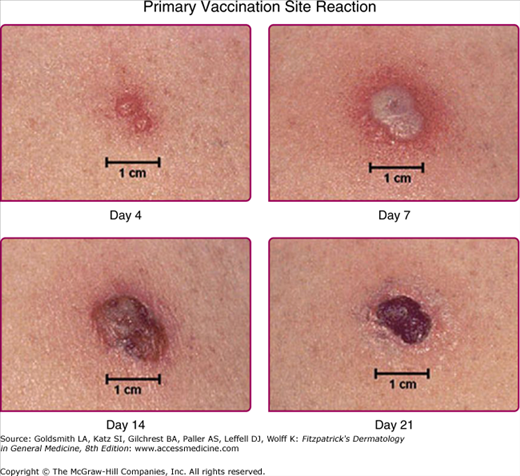
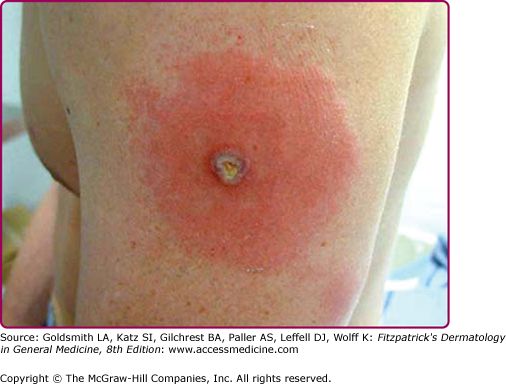
The normal local skin reaction to vaccination begins 3–5 days after administration, starting with a papule that then develops into a vesicle (Jennerian vesicle) followed by a pustule around day 7–9. It crusts and scabs over at day 10–14, with the scab detaching at day 17–21 and leaving a residual scar (Fig. 195-4). A robust take, in which the local reaction is 10 cm or larger in diameter, occurs in 2%–16% of first-time vaccinees (see eFig. 195-4.1). This can be mistaken for bacterial cellulitis, but it occurs 8–10 days after vaccination and self-improves without antibiotic therapy in 24–72 hours.32 In contrast, secondary bacterial infections usually occur within the first 5 days or 30 days after vaccination.
Previously vaccinated individuals have a milder reaction with an accelerated time course. Those with substantial residual immunity may have only erythema with revaccination. Minor local reactions that can occur near the primary site include nearby satellite lesions that progress at the same rate, lymphadenopathy/lymphangitis, and intense surrounding erythema or edema.12
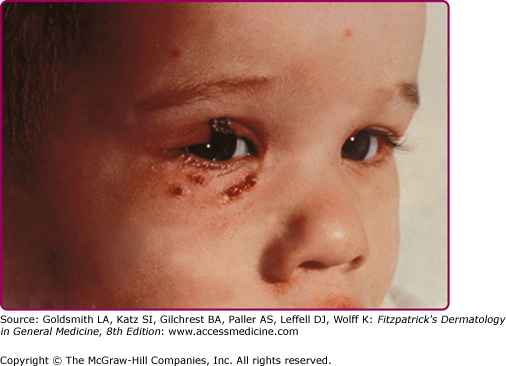
Adverse cutaneous reactions associated with smallpox vaccination can be localized or generalized. Secondary bacterial infection, usually by Staphylococci or group A Streptococci, can occur at the primary site. Accidental vaccinia is the autoinoculation of vaccinia virus from the vaccination site to another area. It is the most common adverse event seen and accounts for about half of all adverse events. The most common sites of transfer are the eyelids (see eFig. 195-4.2), nose, mouth, and genitalia. Lesions are seen at these areas 7–10 days after vaccination, and they usually follow the time course of the original primary lesion. The lesions may be more attenuated if autoinoculation occurs more than 5 days after vaccination as the host immune response is developing.33 Accidental inoculation to the eyes can lead to conjunctivitis, keratitis, or iritis.
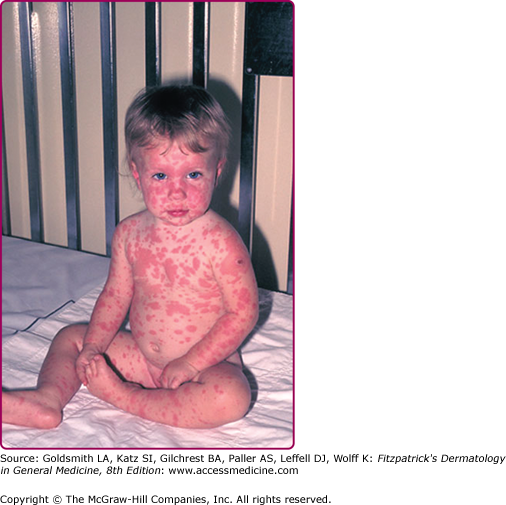
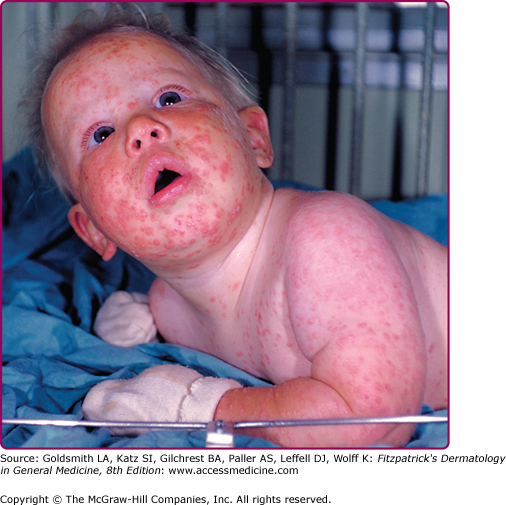
Generalized adverse eruptions can be nonspecific, immune-mediated reactions, including morbilliform and roseola-like rashes. Hypersensitivity reactions such as erythema multiforme can also develop (see eFig. 195-4.3). These rashes typically self-resolve over several days. In rare cases, Stevens–Johnson syndrome can develop and require hospitalization and supportive care. Vaccination can also lead to generalized vaccinia, in which macular, papular, or, less commonly, vesicular lesions can be disseminated to normal skin without evidence of autoinoculation. Generalized vaccinia can be limited or extensive and can occur anywhere on the body (Fig. 195-5). It is thought to be caused by spread of vaccinia virus via the bloodstream and usually occurs 6–9 days after primary vaccination. The lesions progress, as do other vaccinial lesions. The condition is self-limited in immunocompetent individuals but is often more severe in the setting of immunodeficiency.34
eFigure 195-4.3
Erythema multiforme following vaccination in a 1-year-old child. [From the Centers for Disease Control and Prevention (CDC) Public Health Image Library, Atlanta, GA, USA and contributed by Allen W. Mathies, MD and John Leedom, MD of the California Emergency Preparedness Office, Immunization Branch.]
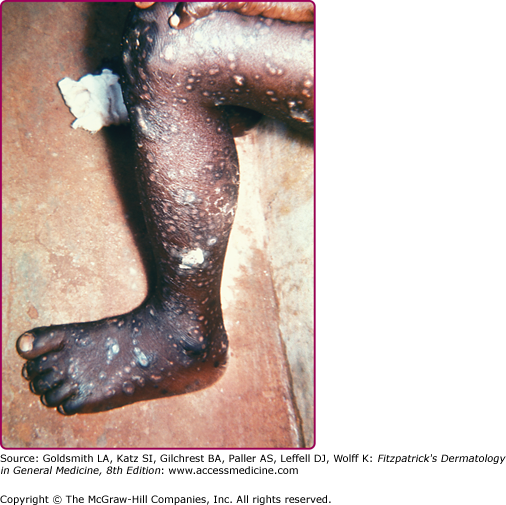
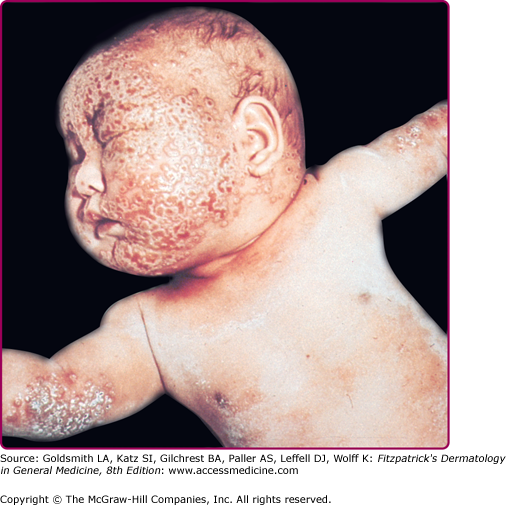
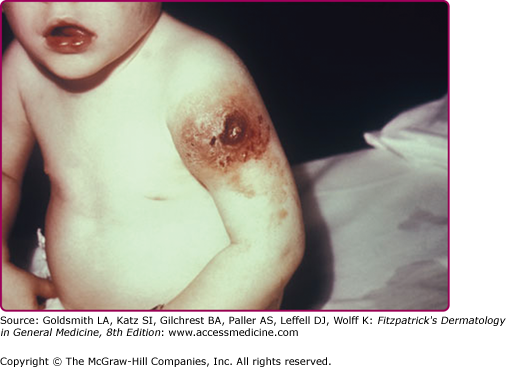
Eczema vaccinatum is the localized or generalized spread of vaccinia virus in individuals affected with atopic dermatitis or, less frequently, other chronic dermatoses such as Darier disease. The individual feels ill with fever, malaise, and lymphadenopathy. It can occur in the primary vaccinee with onset usually at the same time or soon after the appearance of lesions at the vaccination site. Papules, pustules, or vesicles can occur anywhere on the body but have a predilection for areas with prior lesions of atopic dermatitis (see Fig. 195-6). Lesions can range from several to hundreds; the most serious cases result in substantial loss of the skin barrier. The severity of eczema vaccinatum is independent of the severity or activity of the atopic dermatitis or other underlying skin disease. It can also be acquired by secondary transmission, usually by children in contact with a recently vaccinated family member.10,35
Vaccination of individuals with severe impairment of the immune system leads to progressive vaccinia, also called vaccinia necrosa and vaccinia gangrenosa. In these individuals, the primary lesion at the site of vaccination does not heal but instead enlarges and progresses to a painless ulcer with central necrosis (see eFig. 195-6.1). Viral replication is not halted and viremia occurs, with similar metastatic lesions developing at distant sites in the skin, bone, and/or viscera. Most cases have occurred in individuals with defective cell-mediated immunity, but cases have also been described in those with humoral defects.4 The degree of immune impairment may correlate with the risk for development of progressive vaccinia, but the precise protective level is unknown.32
In addition to the skin, other organ systems can be affected by smallpox vaccination. Central nervous system complications can occur in previously healthy individuals. Postvaccinial encephalopathy most commonly affects children younger than 2 years of age, causing cerebral edema without inflammation. Symptoms develop abruptly 6–10 days after vaccination and can include fever, headache, seizures, hemiplegia, aphasia, and transient amnesia. Postvaccinial encephalitis and encephalomyelitis occur 11–15 days after vaccination, with fever, malaise, vomiting, and headache that progress to confusion, seizures, amnesia, spinal cord signs, and coma.36 These complications are thought to be autoimmune reactions, because vaccinia virus is not found in sampled cerebrospinal fluid or tissue. Higher rates of central nervous system complications are seen with non-NYCBH vaccinia strains. Other neurologic events reported to occur in temporal association with smallpox vaccination include transverse myelitis, paralysis, seizures, and polyneuritis, but causality has not been established.2,37
Cardiac events, including myopericarditis, can also occur with vaccination. With the Dryvax vaccine, the rate of myopericarditis among US military vaccinees was elevated 3.6-fold compared to the rate in unvaccinated personnel.38 In clinical trials with ACAM2000, suspected cases of myopericarditis were noted at an approximate rate of 1 in every 175 primary vaccinees, with no cases in revaccinees.28
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree