Site
Menopausal statusa
Observation
Significanceb
Reference
Water barrier function (TEWL, g/m2·h)
Forearm
Premenopausal
3.7 ± 0.4
p < 0.05
Elsner and Maibach [6]
Postmenopausal
2.6 ± 0.3
Vulva
Premenopausal
14.8 ± 1.5
n.s.
Postmenopausal
13.5 ± 1.8
Friction coefficient, μ
Forearm
Premenopausal
0.49 ± 0.02
p < 0.05
Elsner et al. [7]
Postmenopausal
0.45 ± 0.01
Vulva
Premenopausal
0.60 ± 0.04
n.s.
Postmenopausal
0.60 ± 0.06
Hydrocortisone penetration (% dose absorbed)
Forearm
Premenopausal
2.8 ± 2.4
n.s.
Oriba et al. [8]
Postmenopausal
1.5 ± 1.1
Vulva
Premenopausal
8.1 ± 4.1
p < 0.01
Schagen van Leeuwen et al. [9]
Postmenopausal
4.4 ± 2.8
Testosterone penetration (% dose absorbed)
Forearm
Premenopausal
20.2 ± 8.1
n.s.
Oriba et al. [8]
Postmenopausal
14.7 ± 4.2
Vulva
Premenopausal
26.7 ± 8.0
n.s.
Postmenopausal
24.6 ± 5.5
Visual erythema scores (scored on day 2 after 24-h postexposure to 1 % sodium lauryl sulfate)
Forearm
Premenopausal
9
p = 0.03
Elsner et al. [10]
Postmenopausal
5
Vulva
Premenopausal
0
n.s.
Postmenopausal
0
Skin penetration of hydrocortisone and testosterone also has been compared on the forearm and on the vulva. (For perspective, penetration of testosterone but not hydrocortisone is mediated by androgen receptors.) In young women, vulvar skin is more permeable to hydrocortisone than forearm skin; however, following menopause, skin penetration of this steroid drops on the forearm but not on the vulva. By contrast, comparable testosterone penetration rates were measured at both sites in younger women, and menopause had no impact on testosterone penetration at either site [8].
Studies with the model irritant, sodium lauryl sulfate, revealed differences in susceptibility to skin irritation between exposed skin and vulvar skin. Forearm skin was far more susceptible to the model irritant, aqueous sodium lauryl sulfate (1 % w/v): this agent caused intense erythema on the forearms of premenopausal women but no visually discernible response on the vulva in either pre- or postmenopausal women [10].
27.3 Vulvovaginal Atrophy
Vulvovaginal atrophy often develops as hormonal stimulation declines through the menopausal transition (reviewed in [11, 12]). Reportedly, 10–50 % of postmenopausal women exhibit clinical signs and symptoms (Table 27.2) [14–16]. Pubic hair becomes sparse, the labia majora lose subcutaneous fat, and the labia minora and vestibule atrophy [17, 18]. In addition, the introitus narrows, the clitoral hood may become phimotic, and the exposed glans clitoris may fibrose. At the cytological level, estrogen-induced parakeratosis of vulvar stratum corneum, which is highest in the third decade of life, is rarely observed by the eighth decade [19].
Table 27.2
Signs and symptoms of urogenital atrophy
Signs | Symptoms | |
|---|---|---|
Vulvar changes | Sparse pubic hair | Itching, burning, soreness |
Shrunken labia | ||
Inelastic labial skin | ||
Introital narrowing or stenosis | ||
Peri-introital lacerations | ||
Phimotic clitoral hood | ||
Fibrosed glans clitoris | ||
Irritation of the posterior fourchette | ||
Vaginal changes | Smooth, pale, friable vaginal mucosa | Vaginal dryness |
Limited vaginal secretions | Coital discomfort or dyspareunia | |
Vaginal pH >4.5 | Malodorous discharge (in cases of infection) | |
Higher proportion of immature basal cells on Pap smear | Burning leukorrhea (desquamative inflammatory vaginitis) | |
Urinary changes | Eversion of urethral mucosa | Urinary frequency |
Ecchymoses | Dysuria | |
Nocturia | ||
Urinary tract infection |
Vaginal changes also ensue. The vaginal vault becomes shorter and narrower, losing its typical folds (rugae). Blood flow decreases and vaginal lubrication declines. As the epithelium thins, it becomes susceptible to friction-induced bleeding. Moreover, the loss of a glycogen-rich environment both disfavors colonization by lactic acid-producing microbes [20] and reduces hydrogen ion production by vaginal epithelial cells [21, 22]. Consequently, vaginal pH rises above 4.5, which heightens susceptibility to vaginal infection. A Papanicolaou smear of the upper third of the vagina reveals a higher proportion of parabasal cells and lower levels of superficial squamous cells [23–25].
Genital symptoms include decreased vaginal secretions, vaginal irritation, vulvar pruritus, dyspareunia, and postcoital bleeding [12]. Urinary symptoms include urethral discomfort, increased frequency, and dysuria [12]. If the vulvovaginal microbiota becomes disturbed, malodorous vaginal discharge, vulvovaginal inflammation, or recurrent urinary tract infection may accompany atrophic changes. In the patient free of infection, a vaginal pH of greater than 5 is a sign of hypoestrogenism [20, 23].
Only about 25 % of women who experience symptoms mention them to their health-care provider, as many consider their discomfort to be an inevitable consequence of aging. However, urinary pain, vulvovaginal irritation, or dyspareunia secondary to vaginal atrophy may prompt a woman to seek treatment. Low-dose, intravaginal estrogen therapy ameliorates vulvovaginal atrophy without significant systemic side effects and is the conservative and recommended choice when hormone supplementation is considered solely for the relief of this condition [26, 27]. Randomized trials of an ultralow-dose, 10-μg estradiol tablet demonstrated efficacy in normalizing vaginal pH and vaginal cytology and in reducing the most bothersome symptoms of atrophy [28, 29]. This dose exhibited low overall systemic absorption and was associated with no significant evidence of endometrial hyperplasia [28]. The North American Menopause Society concludes that opposing progestogen is generally not indicated at the low estrogen doses administered locally for vaginal atrophy [26, 27].
Intravaginal administration of dehydroepiandrosterone (DHEA), an androgenic sex steroid precursor, has been proposed as an alternative approach to treating postmenopausal vaginal atrophy and associated sexual dysfunction [30, 31]. Locally applied DHEA is converted by vaginal cells to estrogens and androgens without affecting serum concentrations of estradiol or testosterone, thereby avoiding effects on other organs [32]. In randomized trials, treatment improved clinical signs of atrophy (pH, cytology, vaginal secretions, and epithelial thickness) and measures of sexual health [30, 31]. However, in contrast to intravaginal estradiol therapy, which requires application of a tablet two to three times a week, DHEA requires daily dosing of a cream preparation, a regimen that women may find more onerous [33]. The North American Menopause Society (NAMS) Web site (menopause.org) is a good source of information on treatment options for health conditions associated with menopause.
27.4 Urogenital Infections
27.4.1 Urinary Tract Infections
Escherichia coli is the primary organism involved in urinary tract infections (UTIs), and the vagina is a reservoir for urethral colonization [34–36]. As circulating estrogen declines, cell densities of lactic acid-producing microbes fall, and the incidence of vaginal colonization with E. coli rises [37]. This elevates the risk of UTIs. Insulin-dependent diabetes and a prior history of recurrent UTIs are associated with higher risk of UTIs after menopause [38]. Although the number of studies is limited [39, 40], a meta-analysis found evidence that intravaginal estradiol therapy may reduce the risk of urinary tract infection [41]. However, the therapy does not have regulatory approval in the USA for this indication.
27.4.2 Sexually Transmitted Infections
Many people remain sexually active in old age and can be at risk of acquiring sexually transmitted infection. Transmission of genital herpes simplex remains pertinent, and both women with an intact cervix and women who have undergone a hysterectomy can acquire trichomonadal, gonorrheal, or chlamydial infection. With advent of modern treatment, HIV/AIDS is now a chronic illness in the developed world; infected people can now live into their 70s or longer and may transmit the disease. The use of condoms should be encouraged. Sexually transmitted infection among older adults is a sensitive subject that may be facilitated in the clinical setting with a nonthreatening conversation and by using patient education pamphlets.
27.4.3 Desquamative Inflammatory Vaginitis (DIV)
Women over age 40 can suffer from desquamative inflammatory vaginitis, a rare inflammatory vaginal infection that occurs primarily in white women [42, 43]. It produces a copious, purulent discharge, and the vulva and vaginal vault appear glazed due to epithelial sloughing. Vaginal pH is greater than 4.5, but the “whiff test” is negative (no fishy odor when a drop of vaginal secretion is added to 10 % aqueous potassium hydroxide). Microscopy reveals an outpouring of inflammatory white cells (a hallmark sign), a paucity of lactobacilli, large numbers of other bacteria, and a preponderance of immature, squamous vaginal epithelial cells. Typical treatment is a 2-week course of clindamycin. Prognosis is good if there is a favorable initial response, but in some cases, long-term maintenance therapy is required.
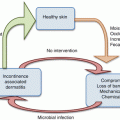
27.5 Urinary Incontinence
Urinary incontinence is underreported condition. It may emerge prior to menopause, but incidence increases with age. Reported prevalence rates vary depending on the condition (stress, urge, or mixed incontinence) and demographic variables (age, race, parity, body mass index, etc.) [44]. Reported rates range from 10 to 40 % among subgroups of community-dwelling individuals [45, 46] and from 43 to 77 % among nursing home residents [47].
Stress and urge incontinence have different symptoms and underlying pathology (Table 27.3). Stress incontinence involves the uncontrolled loss of urine induced by sudden pressure on the abdominal organs (such as a cough, a sneeze, heavy lifting, exercise, or coital penetration). It stems from a weakened sphincter at the junction of the bladder and urethra. Stress incontinence may be first experienced by younger women aged 30–50 when the bladder sphincter is weakened by childbirth. However, its incidence peaks during the perimenopausal period (between the ages 45 and 49) [46], possibly because aging manifests the underlying weakness. Another important factor is obesity, which places additional stress on the bladder. Obese women (BMI ≥30) have twice the risk of developing stress incontinence independent of age and parity. Epidemiological data indicate that each 5-unit increase in BMI is associated with 20–70 % increase in urinary incontinence risk [48].
Table 27.3
Types of urinary incontinence in adult womena
Stress | Urge | |
|---|---|---|
Patient population | Women aged 30–50, especially those who have given birth. Incidence rises with age | Older, usually postmenopausal women (>age 50) |
Symptoms | Uncontrolled urine loss when sudden pressure is applied to the bladder (e.g., sneezing, coughing, lifting heavy objects, intercourse) | Increased frequency and urgency Inability to suppress urine loss |
Causes | Weakness of the sphincter muscle at the junction of the bladder neck and the urethra | Overactive bladder muscle (i.e., stronger, more frequent bladder contractions at lower urine volumes); weakened outlet |
Risk factors | Childbirth; obesity; genital prolapse; Caucasian race | Childbirth may contribute to the problem in younger women by weakening the outlet; the risk after age 50 is independent of childbearing history and may reflect age-related changes |
Mechanism | Nerve or sphincter muscle damage or damage to the connective tissue supporting the bladder neck | Impaired nerve-brain reflexes regulating bladder wall contractions; shortening and thinning of the urethra after menopause; slower and less efficient voiding (retention). Childbirth may weaken the outlet, making the impact of bladder contractions more apparent |
Treatment | Pelvic floor muscle training (Kegel exercises) | Behavioral modifications |
Surgery (least conservative) | Antimuscarinic drugs |
Urogenital prolapse, the downward descent of the internal urogenital organs toward the vagina due to weakened support, can coexist with stress incontinence. Relaxation of the musculature of the vaginal vault and weakening of the pelvic muscles due to childbirth contributes to urogenital prolapse.
Mild stress incontinence is manageable with pelvic muscle training (Kegel exercises), by limiting fluid intake, by more frequent voiding, and with the use of feminine pads. Pelvic floor muscle exercises are more effective in younger than older women [49]. Devices for stress incontinence include pessaries and urethral orifice plugs, but most women are disinclined to use them. Duloxetine, a serotonin and noradrenaline reuptake inhibitor used to treat mood disorder, reduces the frequency of stress incontinence in randomized controlled trials in younger and postmenopausal women [9, 50, 51]. It is approved for this indication in Europe but not in the USA. In overweight or obese women, weight loss is a first-line intervention for stress urinary incontinence. For severe cases of stress incontinence, surgery is the least conservative remaining option for therapy; however, a paucity of data exists on the effectiveness of surgery in the older postmenopausal patient.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree