Fig. 17.1
Differences in structure between young and aged skin (Reproduced with permission from Farage [6])
17.2.1 Epidermis
This outer layer of the skin is composed primarily of keratinocytes, which account for ~90 % of the cells [1]. Melanocytes, Langerhans cells, and Merkel cells are also found. Thickness varies according to the individual and the anatomic site and averages from 50 to 100 mm (as reviewed by Farage et al. [2]). The epidermis fulfills two main functions: protecting the skin from external insult and maintaining hydration of internal tissues. Both functions are accomplished primarily by the outermost layer of the epidermis, the stratum corneum (SC). In healthy adults, water content of the viable portion of the epidermis is maintained at about 70 % [7].
Keratinocytes originate in a single layer of cells at the basement membrane (the layer between the dermis and the epidermis) and move upward. As they ascend and mature, they produce keratin and lipids, and the morphology alters. By the time they reach the SC, they are flattened cell bodies of keratinocytes, now called corneocytes. The SC averages 15 layers over most of the body but varies widely depending on body site. One body site with the fewest number of layers is the genital skin (6 ± 2), followed by the face (9 ± 2), neck (10 ± 2), scalp (12 ± 2), trunk (13 ± 4), and extremities (15 ± 4) [8]. In the thick palmoplantar skin of the palms and sole, the SC is in excess of 50 layers [8].
Corneocytes of the SC are covered by a highly cross-linked and cornified envelope, with strongly adhering lipids, i.e., ceramides, long-chain free fatty acids, and cholesterol [9]. These intercellular lipids, as well as sebum, natural moisturizing factor (NMF), organic acids, and inorganic ions, impart the water-holding capacity of the SC. The SC is comprised of about 50–60 % structural proteins, 20–25 % water, and 15–20 % lipids [10]. When the barrier function and water-retaining capacity of the SC are compromised, pathologic skin dryness can develop [11]. The skin is considered clinically dry when the moisture content of the SC falls below 10 % [12]. Dry skin is less flexible and is subject to cracking or fissuring.
17.2.1.1 Overall Structural Changes
Changes that occur to the epidermis with aging are summarized in Table 17.1. An important overall change is a thinning of the epidermis. Starting at about age 30, the epidermis decreases in thickness at about 6.4 % per decade [15]. Changes in epidermal thickness are most pronounced in exposed areas, such as the face, neck, upper part of the chest, and the extensor surface of the hands and forearms [34]. One contributor to the overall thinning of aged skin is a flattening of the dermal–epidermal junction due to a retraction of the rete pegs [15]. As a consequence of the reduced interdigitation between the dermis and epidermis, the skin becomes less resistant to shearing forces and more vulnerable to insult [20]. Furthermore, flattening of the dermal–epidermal junction results in a smaller contiguous surface between the two layers and reduces the supply of nutrients and oxygen to the epidermis [16]. This flattening also may limit basal cell proliferation, affect percutaneous absorption [15], and contribute to wrinkle formation [20].
Table 17.1
Structural and compositional changes that occur in aging skin
Observed effect of aging | References | |
|---|---|---|
Epidermis | ||
Overall structural changes | Decrease in overall thickness | [13] |
Dermal–epidermal junction flattens | [14] | |
Retraction of rete pegs | [15] | |
Dermal papillae decrease in number and size | [16] | |
Cellular changes | Epidermal cell numbers decrease | [17] |
Epidermal turnover rate decreases and morphology is less homogeneous | [18] | |
Cells of the basal layer become less uniform in size | [19] | |
Keratinocytes become shorter and fatter | [17] | |
Corneocytes are fewer and larger | ||
Enzymatically active melanocytes decrease in number | [24] | |
Langerhans cells in the epidermis decrease in number and display a more heterogeneous appearance | ||
Compositional changes | Water content of aged skin is lower than that of younger skin | |
The total lipid content decreases, particularly: ceramide 1 linoleate, ceramide 3, triglycerides, and the sterol ester fraction | ||
Reduced amount of cutaneous NMF | [11] | |
Dermis | ||
Overall structural changes | Decrease in overall thickness | [15] |
Structure of sweat glands becomes distorted, number of functional sweat glands decreases | [27] | |
Number of blood vessels decreases | [28] | |
Cellular changes | Fibroblasts decrease in number | [28] |
Mast cells decrease in number | [28] | |
Number of nerve endings is reduced | [4] | |
Pacinian and Meissner’s corpuscles degenerate | [29] | |
Compositional changes | Collagen synthesis decreases, and fibers and bundles are altered | |
Elastic fibers degrade | [12] | |
Hyaluronic acid and glycosaminoglycans are depleted, reducing water-holding capacity | ||
Interfibrillary ground substance decreases | [32] | |
Hypodermis | ||
Overall volume decreases | [28] | |
Distribution of subcutaneous fat changes | [33] | |
17.2.1.2 Cellular Changes
With aging, epidermal cell numbers and the epidermal turnover rate decrease. The capacity for re-epithelialization diminishes [35]. Characteristic changes occur in each of the cell types in the epidermis (see Table 17.1). Basal layer cells, keratinocytes, and corneocytes decrease in number and become less uniform in size. A decrease in enzymatically active melanocytes results in uneven pigmentation in elderly skin, and a decrease in Langerhans cells leads to impairment of cutaneous immunity.
17.2.1.3 Compositional Changes
Water content of the SC decreases progressively with age and eventually falls below the level necessary for effective desquamation. This causes corneocytes to pile up and adhere to the skin surface, which accounts for the roughness, scaliness, and flaking that accompanies xerosis in aged skin.
Integrity of the SC barrier is dependent on an orderly arrangement of critical lipids [36]. The total lipid content of the aged skin decreases dramatically, and this alteration in the lipid barrier results in dryer skin [37]. Age-related changes in the amino acid composition [12] reduce the amount of cutaneous NMF, thereby decreasing the skin’s water-binding capacity [11].
17.2.2 Dermis
The dermis is a dense and irregular layer of connective tissue 2–3 mm thick that comprises most of the skin’s thickness (see Fig. 17.1) [30]. The three major extracellular components of the dermis are collagen (which provides tensile strength), elastin (which provides elasticity and resilience), and hyaluronic acid (which provides water-holding capacity). Approximately 80 % of the dry weight of adult skin consists of collagen, and about 5 % of the dermis consists of elastin [30]. The dermis also contains much of the skin’s vasculature, nerve fibers, and sensory receptors.
17.2.2.1 Overall Structural Changes
As with the epidermis, overall dermal thickness decreases with age at the same rate in both genders [12] (see Table 17.1). Loss of dermal collagen and elastin makes up most of the reduction in total skin thickness in elderly adults. The number of functional sweat glands and blood vessels decreases with aging.
17.2.2.2 Cellular Changes
Cellularity of the dermis generally decreases with aging as the number of fibroblasts and mast cells decreases. Perception of pressure and touch also decreases in aged skin as the number of Pacinian and Meissner’s corpuscles degenerates.
17.2.2.3 Compositional Changes
All three major extracellular components of the dermis (collagen, elastin, and hyaluronic acid) are depleted in older skin. Collagen content decreases at about 2 % per year [18], due to both a decrease in collagen synthesis [28] and an increase in the degradation of collagen [38]. The relative proportions of collagen types are also disrupted in aged skin. The proportion of type I collagen to type III collagen in young skin is approximately 6:1, a ratio which drops significantly over the lifespan as type I collagen is selectively lost [39] and type III synthesis increases [40]. Collagen fibers become thicker and collagen bundles more disorganized than in younger skin [18]. Collagen cross-links stabilize resulting in a loss of elasticity.
Functional elastin declines in the dermis with age. Elastin becomes calcified, elastin fibers degrade [34], and turnover declines [28]. The amount of glycosaminoglycans (GAGs), an important contributor to the structure and water-holding capacity of the dermis, declines with age, as does the amount of hyaluronic acid produced by fibroblasts [16].
Loss of structural integrity of the dermis leads to increased rigidity and diminished elasticity [31], with a concomitant increase in vulnerability to shear force injuries [20]. These properties erode faster in women than in men [30]. The impact of these changes is dramatic: for example, when the skin is mechanically depressed, recovery occurs in minutes in young skin but takes over 24 h in skin of aged individuals [18].
17.2.3 Hypodermis
Hypodermis is a layer of loose connective tissue below the dermis (see Fig. 17.1). It contains the larger blood vessels of the skin, subcutaneous fat (for energy storage and cushioning), and areolar connective tissue. The hypodermis provides cushioning, insulation, and thermoregulation, and it stabilizes the skin by connecting the dermis to the internal organs.
Hypodermis loses much of its fatty cushion with age (see Table 17.1). The basement membrane, a very small fraction of the total skin thickness, actually increases in thickness with age [41]. Overall volume of subcutaneous fat typically diminishes with age, although the overall proportion of subcutaneous fat throughout the body increases until approximately age 70. Fat distribution changes as well, that is, in the face, hands, and feet, decrease, while a relative increase is observed in the thighs, waist, and abdomen. Physiological significance may be to increase thermoregulatory function by further insulating internal organs.
17.3 Functional and Physiological Changes in the Skin with Aging
Physiological changes that occur in aged skin are summarized in Table 17.2 and include changes in barrier function and permeability, vascularization and thermoregulation, irritant response, regenerative capacity and response to injury, immune response, biochemical changes, and neurosensory perception.
Table 17.2
Functional and physiological changes that occur in aging skin
Observed effect of aging | References |
|---|---|
Barrier function and permeability | |
Lower baseline TEWL | [42] |
Altered percutaneous absorption | [43] |
Decreased vascularization | [44] |
Decreased chemical clearance | [18] |
Decreased sebum production | [18] |
Increased vulnerability to mechanical trauma | [18] |
Vascularization and thermoregulation | |
Loss of vascularization | |
Maximum level of blood flow diminished | [45] |
Autonomic vasoconstriction delayed | |
Decreased sweat production | [46] |
Irritant response and regenerative capacity | |
Lower inflammatory response (erythema and edema) | [47] |
Altered response to irritants that elicit inflammation by different mechanisms | |
Attenuated response to sunburn | [20] |
Decrease in inflammatory cells | [20] |
Renewal time of stratum corneum increased by 50 % | [18] |
Decreased wound healing | [18] |
Reduced reepithelialization | [18] |
Immune response | |
Decreased number of Langerhans cells | [20] |
Decreased number of circulating thymus-derived lymphocytes | [20] |
Decreased risk and intensity of delayed hypersensitivity reactions | [50] |
Biochemical changes | |
Increase in pH after about age 70 | [15] |
Decreased vitamin D production | [51] |
Reduced elasticity | [31] |
Sensory and pain perception | |
Increased itching | [52] |
Loss in sensitivity, especially after age 50 | [20] |
17.3.1 Barrier Function and Permeability
Baseline transepidermal water loss (TEWL), a measure of the functional capacity of the SC to maintain the moisture content of the skin, is lower in older patients compared to younger indicating a reduced capacity of the SC to maintain the moisture content of the skin [13, 48]. Recovery of baseline TEWL values after occlusion is also impaired in older skin [42].
Permeability of the skin is altered with aging. Penetration of permeants through the skin involves (1) absorption to the stratum corneum; (2) diffusion through the stratum corneum, epidermis, and papillary dermis; and (3) removal by microcirculation [13]. The first two steps depend on the integrity and hydration of the stratum corneum, which in turn is a function of the level and composition of intracellular lipids [13]. The final step depends on the integrity of the microcirculation [13]. Studies on percutaneous absorption in the aged have produced conflicting results (as reviewed in Farage et al. [2]) that may reflect compound- and body-site differences in the rates of percutaneous absorption. Epidermal penetration of a substance is strongly associated with its hydrophobicity relative to the lipid content of the skin. Because aged skin is drier and has a lower lipid content than younger skin, it may be less amenable to penetration by hydrophilic moieties [43]. Diffusion to the dermis may be compromised by the flattening of the dermal–epidermal junctions. Reduced vascularization in older skin would impact the removal of penetrants by the microcirculation.
Permeability barrier of aged skin is more vulnerable to disruption. In a study in which loss of barrier integrity was achieved by tape-stripping, adults over 80 required only 18 strippings as compared to 31 strippings in young and middle-aged adults [53]. In the aged subjects, the time for recovery of barrier function was also dramatically increased. At 24 h, only 15 % of the older subjects had returned to baseline TEWL compared to 50 % of the younger group. Artificially induced water gradients produced by occlusion dissipated more slowly in older skin [42].
17.3.1.1 Vascularization and Thermoregulation
Overall, vascularity is lost in aged skin. Capillaries and small blood vessels regress and become more disorganized [20], blood vessel density diminishes [15], and a 30 % reduction in the number of venular cross sections per unit area of the skin surface occurs in non-exposed areas of the skin [20]. Capillaroscopic measurements using fluorescein angiography and native microscopy suggest a decrease in dermal papillary loops, which house the capillary network [15]. Although the pattern of blood flow through individual capillaries remains unchanged [45], the maximum level of blood flow diminishes as functional capillary plexi are lost.
A significant time delay in autonomic vasoconstriction in the aged (e.g., after postural changes, cold arm challenge, inspiratory gasp, body cooling) [13, 15] is well documented; this phenomenon is due primarily to declining function of the autonomic nervous system [13].
Eccrine sweating markedly decreased with age. Spontaneous sweating in response to dry heat was 70 % lower in healthy older subjects compared to young controls, due primarily to decreased output per gland [46].
17.3.1.2 Irritant Response and Regenerative Capacity
Inflammatory response to exogenous agents declines in people over 70 years old [13, 48]. It is slower and less intense, and some clinical signs of skin damage are absent [13, 47]. A comprehensive review of clinical assessments of the erythematous response in older people suggests that susceptibility to skin irritation generally decreases with age [54]. For example, a compilation of results of skin patch tests conducted among older people over a period of 4 years demonstrated a trend toward lower reactivity to four common irritants with age [55]. Specifically, older people exhibited significantly lower reactivity to two strong irritants (20 % sodium dodecyl sulfate and 100 % octanoic acid) and directionally lower reactivity (approaching statistical significance) to two milder irritants (100 % decanol and 10 % acetic acid). Elderly adults were less reactive to a range of skin irritants that elicit inflammation by clearly different mechanisms (references provided in Table 17.2). In addition, people aged 65–84 years were less reactive to stinging caused by 5 % sodium lauryl sulfate (SLS) than people aged 18–25 years [56].
Pretreatment with 0.25 % SLS also had less effect on skin barrier function in elderly people (mean age, 74.6 years) compared to younger adults (mean age, 25.9 years) [57]. Analysis of changes in TEWL after sodium lauryl sulfate (SLS) application to the skin confirmed that in aged skin, the irritation reaction is slower in postmenopausal women compared to premenopausal women [58]. Moreover, although blistering caused by ammonium hydroxide exposure is elicited more rapidly in older people, the time required to attain a full response is much longer than in younger ones [13].
Barrier function requires twice as long to restore in the aged as compared to younger individuals [59]. Repair of an impaired barrier requires the presence of the three main lipids in appropriate proportions, i.e., ceramides, cholesterol, and free fatty acids [60], as well as stratum corneum turnover; both of which are suboptimal in older subjects. In healthy skin, about one layer of corneocytes desquamates every day, so that the whole stratum corneum replaces itself about every 2 weeks [9]. In contrast, elderly stratum corneum may take twice as long [61].
Injury repair diminishes with age. Wound healing events begin later and proceed more slowly. For example, a wound area of 40 cm2, which in 20-year-old subjects took 40 days to heal, required almost twice as long – 76 days – in those over 80 [18]. The risk of postoperative wound reopening increased 600 % in people in their mid-80s compared to those in their mid-30s [18]. Tensile strength of healing wounds decreased after the age of 70. Repair processes like collagen remodeling, cellular proliferation, and wound metabolism are all delayed in the aged. The rate at which fibroblasts initiated migration in vitro following wound initiation was closely related to the age of the cell lines [18].
17.3.1.3 Immune Response
The immune response of aged skin is generally diminished. Numbers of Langerhans cells in the epidermis decrease by about 50 % between the age of 25 and the age of 70 [18]. Total number of circulating lymphocytes decreases, as does the number of T cells and B cells [18], both of which lose functional capacity with age [62].
Delayed hypersensitivity reactions decrease with age: numerous reports have demonstrated a decrease in the capacity for allergic response [18, 50]. For example, healthy older subjects did not develop sensitivity to some known sensitizers and exhibited a lower frequency of positive reactions to standard test antigens compared to young adult controls [18].
17.3.1.4 Biochemical Changes
The surface pH of normal adult skin averages pH 5.5. This cutaneous acidity discourages bacterial colonization and contributes to the skin’s moisture barrier as amino acids, salts, and other substances in the acid mantle absorb water [63]. The pH of the skin is relatively constant from childhood to approximately age 70 [15] then rises significantly. This rise is especially pronounced in lower limbs, possibly due to impaired circulation.
Vitamin D content of aged skin declines: synthesis of this compound slows because the dermis and epidermis have significantly reduced levels of its immediate biosynthetic precursor (7-dehydrocholesterol), which limits formation of the final product [51].
17.3.1.5 Neurosensory Perception
Itching is reported more frequently by older adults. However, pain perception declines and is delayed after age 50 [20]. Consequently, the risk of tissue injury rises, as the most obvious warning signals – pain, erythema, and edema – appear more slowly [20]. This, coupled with longer wound repair times, results in higher morbidity in the aged.
17.4 Skin Sensitivity and Aging
Based on objective assessments in patch tests and sting tests, as reviewed in a previous section, the inflammatory response to exogenous agents declines in the elderly. However, “sensitive skin” is subjective in nature. It is a condition of cutaneous hyper-reactivity that can result in exaggerated reactions to physical factors (e.g., weather extremes), chemical factors (e.g., cosmetics and other consumer products), and/or psychological or hormonal factors (e.g., stress or menstrual cycles) [64, 65]. It is commonly perceived as itching, burning, stinging, tingling, or a tight sensation. Often, there is no outward sign of irritation.
Prevalence of subjective, or self-assessed, sensitive skin in the general population has been the subject of a number of recent investigations using questionnaire-based surveys. Reported prevalence varies depending on the specific study population and the precise nature of the questions posed to the participants. Generally, the reported prevalence is about 40–60 % in the US and in European populations [65–70]. In two recent studies in China, the reported incidence was 15–20 % [71, 72]. One investigator suggested that the lower reported rates in China may be related in part to an unfamiliarity with the term and concept of “sensitive skin” among the Chinese population [72].
We previously reported the perceptions of skin sensitivity overall and at specific body sites among different age groups and genders through a questionnaire-based survey of 1,039 people in 2006 in Midwestern USA [67, 73–76]. Sixty-eight percent of the study population described themselves as having overall sensitive skin to some degree (Table 17.3): 77 % perceived their facial skin to be sensitive, 61 % perceived their body area to be sensitive, and 56 % perceived their genital area to be sensitive. Adverse reactions to products resulting in either visual signs (e.g., redness or swelling) or unpleasant sensations (e.g., burning, stinging, or itching) were cited as the reason for the perception of sensitive skin in about half of the responders. Extreme weather conditions were cited by about a third of the responders.
Table 17.3
Perceptions of sensitive skin
Question: Some people have skin that is more sensitive than others. How would you describe your skin? | Question: Please rate your skin in each of the following areas: | |||
|---|---|---|---|---|
Overall rating of skin sensitivity | Facial area | Body area | Genital area | |
Sensitive (any degree) | 711 (68 %) | 799 (77 %) | 628 (61 %) | 580 (56 %) |
Very sensitive | 51 (5 %) | 111 (11 %) | 19 (2 %) | 88 (9 %) |
Moderately sensitive | 239 (23 %) | 245 (24 %) | 189 (18 %) | 140 (14 %) |
Slightly sensitive | 421 (41 %) | 443 (43 %) | 420 (41 %) | 352 (34 %) |
Not sensitive | 328 (32 %) | 234 (23 %) | 407 (39 %) | 451 (44 %) |
Total number of subjects responding | 1,039 | 1,033 | 1,035 | 1,031 |
Other investigators reported on differences in the prevalence of sensitive skin among different age groups. Guinot et al. (2006) reported a decline in the reported incidence of sensitive skin in older subjects [69]. In a recent study conducted in China, the prevalence of “sensitive” and “very sensitive” skin decreased with age [72]. Misery et al. reported no significant difference between age groups and the proportion of the subjects claiming sensitive skin [65]. In our investigation, we found no age-related trends among subgroups of responding subjects in the perception of sensitive skin overall or sensitive skin at the specific sites of the face or body area (Fig. 17.2 a–c) [76]. However, age-related differences were apparent when asked about sensitivity of the skin of the genital area. The perception of sensitive genital skin rose directionally with age. Among the older subgroup of subjects (aged 50 or older), sensitivity of the skin of the genital area was significantly more likely to be reported compared to subjects in the <30 or 31–39 age subgroups (see Fig. 17.2d).
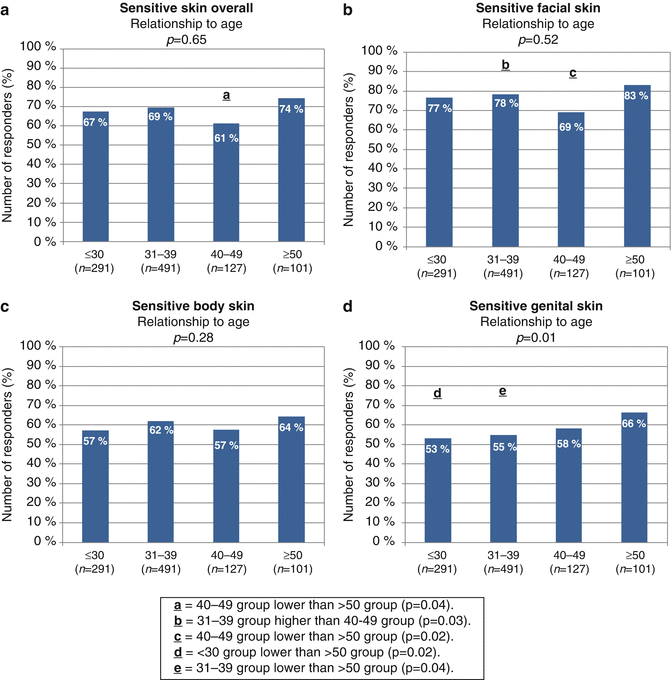

Fig. 17.2
Age group differences in perceptions of sensitive skin. The percentage of participants who claimed some degree of sensitivity overall (a) or sensitivity of the facial, body, or genital areas (b–d). Correlations between perceptions of sensitive skin and age were assessed by MH Chi-square. Paired age group comparisons were performed by Chi-square analysis. (a) On the bar of a – 40–49 group significantly lower than ≥50 group (p = 0.04). (b) On the bar of b – 31–39 group significantly higher than 40–49 group (p = 0.03). (c) On the bar of b – 40–49 group significantly lower than ≥50 group (p = 0.02). (d) On the bar of d – ≤30 group significantly lower than ≥50 group (p = 0.02). (e) On the bar of d – 31–39 group significantly lower than ≥50 group (p = 0.04) (Adapted from Farage [76])
In several investigations, the prevalence of perceived sensitive skin is higher for women compared to men. Generally, the prevalence has been reported as 50–60 % for women and 30–40 % for men [65, 66, 69, 70]. We evaluated gender differences (Table 17.4) and found that the number of women who reported some degree of skin sensitivity overall (69.0 %) was higher than the number of men (64.4 %), but this difference did not reach significance (p = 0.310) [67]. In addition, we found no gender differences in the prevalence of perceived sensitive skin of the face or body (p = 0.474, p = 0.360, respectively). However, a significantly higher proportion of women specifically perceived their genital skin to be sensitive (58.1 % of females and 44.2 % of males) (p < 0.030). An analysis was conducted to determine if there was a significant association between age and perceived sensitive skin. No association was significant with the exception of sensitive skin of the genital area among women (p = 0.012). The association of age and perceived genital skin sensitivity was not significant for men (p = 0.173).
Table 17.4
Perceptions of sensitive skin by age and gender subgroups
Responders with sensitive skin (%) | |||||||
|---|---|---|---|---|---|---|---|
General | Face | ||||||
Females | Males | p value | Females | Males | p value | ||
All responders | Females, n = 869 | 69.0 % | 64.4 % | 0.310 | 78.6 % | 68.1 % | 0.474 |
Males n = 163
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |||||||


