Phototherapy: Introduction
|
Diseases amenable to phototherapy include psoriasis, atopic dermatitis, cutaneous T-cell lymphoma, vitiligo, localized and systemic scleroderma, pruritus, photodermatoses, lichen planus, pityriasis lichenoides, urticaria pigmentosa and granuloma annulare.
Phototherapy is the use of ultraviolet radiation or visible light for therapeutic purposes. Its beneficial effects in vitiligo were first recognized thousands of years ago in India and Egypt, and its activity is now well established for a variety of other dermatologic conditions. The enduring appeal of phototherapy is based on its relative safety coupled with an ongoing interest in its molecular and biological effects. The manufacture of light sources that emit selective wavelengths of radiant energy, identification of photosensitizers with unique photochemical properties, and the development of novel methods for the delivery of light to cutaneous and noncutaneous surfaces have all contributed to its expanded use for dermatologic and nondermatologic conditions. Aside from lasers, high output incoherent light sources, and visible light sources employed for photodynamic therapy, the main phototherapic devices that are in use today are broadband UVB (BB-UVB), narrowband UVB (NB-UVB), UVA1, and UVA for psoralen photochemotherapy (PUVA). Ideally, devices used for therapeutic ultraviolet radiation (UVR) should do so in a safe, efficient and cost-effective manner. Understanding the basic principles of these devices is important for dermatologists and other providers utilizing phototherapy for the management of dermatologic diseases.1–2
Mechanisms of Phototherapy
The different wavelengths of ultraviolet radiation used for phototherapy each have distinct photochemical and photobiologic properties, which include differences in depth of penetration and the range of molecules in the skin with which they interact. As a consequence, each form of phototherapy has unique properties with respect to potency, side effects, and diseases in which they are effective.
Most UVB radiation (290–320 nm) is absorbed by the epidermis and superficial dermis.3 This form of radiant energy produces many different types of DNA damage4; however, pyrimidine dimers and 6,4 pyrimidine-pyrimidone photoproducts are thought to be particularly important for both its efficacy and its toxicity. UVB also causes photochemical changes in trans-urocanic acid, converting it to the cis-form of the molecule. Urocanic acid is a breakdown product of histidine and is present in large amounts in the stratum corneum. Originally considered to be a natural photoprotectant, there is now a substantial evidence that cis-urocanic acid is a mediator of the UVB-induced immunosuppression.5 A third direct target of UVB radiation is the amino acid tryptophan. UVB converts tryptophan into 6-formylindolo[3,2-b]carbazole (FICZ), which binds to the intracellular arylhydrocarbon hydroxylase (Ah) receptor, initiating a series of events that culminates in activation of signal transduction pathways. One such pathway results in expression of cyclooxygenase-2, an enzyme required for synthesis of prostaglandin E2.6 Finally, there is evidence that UVB exposure leads to the generation of reactive oxygen intermediates, which has downstream effects such as DNA damage in the form of 8-oxo-deoxyguanosine, lipid peroxidation, activation of signal transduction pathways and stimulation of cytokine production.7
In contrast to UVB radiation, which has a relatively superficial depth of penetration, UVA radiation (320–400 nm) can reach the mid- or lower dermis.3 It is therefore more effective than UVB for skin diseases in which the cutaneous pathology lies deeper than the superficial dermis. Like UVB, UVA radiation can produce pyrimidine dimers in DNA, but, on a per photon basis, it is much less effective at doing so.8 In most situations, the major biological effects of UVA radiation are due to the generation of reactive oxygen intermediates.9 Following UVA exposure, reactive oxygen intermediates are formed in mitochondrial enzyme complexes during oxidative phosphorylation. Although the skin contains antioxidants, reactive oxygen intermediates formed during phototherapy exceed the amount that can be neutralized by endogenous photoprotective activities. UVA-induced oxidants are capable of harming DNA, lipids, structural and nonstructural proteins and organelles such as mitochondria. The generation of oxidants following UV exposure has been implicated in photoaging of the skin and skin cancer.
In psoralen photochemotherapy, psoralen photosensitizers are activated by UVA radiation, and the depth of penetration of PUVA is the mid-dermis. The major photochemical effect of psoralen photochemotherapy is damage to DNA. The changes in DNA differ from those of UVB and UVA without psoralens.10 Psoralens used for photochemotherapy have two double bonds that can absorb UVA radiation. When administered to an individual, these compounds intercalate with DNA. Following UVA exposure, they form a single adduct with DNA and then become a bifunctional adduct, cross-linking the DNA strands in the double helix when a second photon is absorbed. There is also some evidence that photochemotherapy augments the production of reactive oxygen intermediates such as singlet oxygen. This effect has been implicated in induction of the cyclooxygenase enzyme and activation of arachidonic acid pathways.11
(See also Chapter 90)
The photoimmunological effects of phototherapy are thought to provide an explanation, at least in part, for its efficacy in cutaneous diseases in which T-cell hyperactivity predominates (e.g., psoriasis, atopic dermatitis, lichen planus). Under normal circumstances, both effector and regulatory T-cells are generated, with the overall intensity of the immune response dependent on the relative proportion of effector and regulatory T-cells populations that are present. UVB exposure inhibits activation of effector T-cells, whereas it leaves formation regulatory T-cells unaltered.12 As a result, the equilibrium of effector and regulatory T-cells is biased toward a diminished cell-mediated immune response. This perturbation in the balance of effector and regulatory T-cells reflects disruption of the activities of dendritic cells within the skin, the major function of which is to present antigen to T-lymphocytes. This is due to direct effects of UVB on dendritic cells and indirectly through the production of IL-10 and prostaglandin E2, both of which have been shown to diminish the capacity of dendritic cells to present antigen to effector T-cells and to suppress T-cell responses.13 Increased levels of IL-10 have been found after UVB, UVA1, and PUVA exposure. PGE2 production occurs through UVB effects on keratinocytes14–16; UVB is an inductive stimulus for cyclooxygenase-2, which is important for PGE2 production. Other immunosuppressive soluble mediators that have been reported to be increased following UVB exposure including agonists of the platelet-activating factor receptor,17 MSH, and calcitonin gene-related peptide.18
PUVA has effects that are similar to UVB with respect to antigen presenting cells within the skin, the balance between effector and regulatory T-cells, and the production of soluble immunosuppressive mediators.12 There is limited information on the effect of UVA1 on antigen presenting cells and on effector and regulatory T-cells.
In addition to its actions on cutaneous antigen presenting cells, phototherapy causes cell death by apoptosis in T-cells present in cutaneous lymphoid infiltrates. This has been demonstrated for UVA1 phototherapy in the lymphocytic infiltrate in atopic dermatitis,19 and for narrowband UVB in psoriasis.20
Another immunological effect of phototherapy is on expression of ICAM-1 (CD54) and other adhesion molecules. ICAM-1 is not normally present on epidermal keratinocytes, but can be induced in a variety of inflammatory skin conditions. It facilitates T-cell binding to keratinocytes, through its interaction with LFA-1 that is present on T-cells. UVB, UVA1, and PUVA have all been shown to interfere with keratinocyte expression of ICAM-1, and this effect of phototherapy may therefore contribute to its efficacy in diseases, which have increased keratinocyte ICAM-1 expression.12
Both UVA1 and PUVA have deleterious effects on mast cells, although the mechanisms of action differ.21–22 PUVA is not cytotoxic for mast cells, and because of this the reduction in mast cell concentrations in the dermis of PUVA-treated skin is relatively small. However, it does stabilize mast cell membranes and in so doing limits the release of histamine and other mediators when these cells are stimulated to degranulate.22 In contrast, chronic therapy with UVA1 results in apoptosis of mast cells with a marked reduction in their concentrations in the dermis, which can last for several months.20 Both PUVA and UVA1 have been employed to treat selective mast cell–mediated diseases.
One of the downstream effects of UVA-induced generation of reactive oxygen intermediates is activation of matrix metalloproteinase-1 (MMP-1),23–24 the major biologic activity of which is degradation of collagen. UVA radiation also increases the production of IL-1 and IL-6, which are stimuli for MMPs.25 PUVA has also been shown to increase MMPs.26 These effects of UVA1 and PUVA on MMP-1 and collagen degradation provide the rationale for its use in sclerotic skin diseases.
UVB, PUVA, and UVA all cause acanthosis of the epidermis and thickening of the stratum corneum.27 This effect accentuates light scattering and increases its absorption by the upper levels of the epidermis. As a consequence, phototherapy treatment doses must be progressively increased so that an equivalent number of photons can reach the lower levels of the epidermis and dermis where therapeutic targets lie. On the other hand, this attribute of phototherapy has been exploited for the management of chronic photosensitivity disorders because this “hardens” the skin, permitting individuals afflicted with these disorders to tolerate greater amounts of sun exposure.
Exposure to ultraviolet radiation is also known to stimulate melanogenesis,28 which is at least in part a consequence of DNA damage and/or its repair.29–32 Experimental studies have shown that treatment of melanocytes with DNA repair enzymes increases the melanin content of melanocytes,33 and application of small fragments of thymidine dinucleotides to guinea pig skin produces a tanning response.30–32 The stimulatory effects on melanogenesis increase the tolerance of patients with some photosensitivity disorders to ambient sun exposure, but also decrease the efficacy of phototherapy unless the doses of ultraviolet radiation are gradually increased.
Narrowband UVB and PUVA are also employed to repopulate vitiliginous skin with melanocytes. The mechanism by which phototherapy stimulates repigmentation of vitiliginous skin is incompletely understood, but may involve stimulation of hair follicle melanocyte proliferation and migration.34 Cytokines and other inflammatory mediators released from other cells, such as keratinocytes, are thought to stimulate inactive melanocytes in the outer root sheath of hair follicles to proliferate, mature, and migrate to repopulate the interfollicular epidermis.35
Phototherapy Apparatuses
In the ideal situation, the wavelengths that are most effective for the treatment (i.e., the action spectrum) for every dermatologic condition would be known and there would be a device capable of delivering those wavelengths specifically to lesional skin. For some skin diseases such as psoriasis, great strides have been made toward this ideal and targeted therapy using devices such as excimer lasers36 and nonlaser devices known as monochromatic excimer light (MEL) devices37 that can deliver wavelengths of UVR at or close to those that are most effective at clearing psoriatic plaques have been evaluated and are being used clinically. Unfortunately, for most dermatologic conditions this information is unknown. However, the increased availability of improved phototherapy devices and novel treatment approaches are providing new options for patients and clinicians. In addition, as more studies are conducted utilizing phototherapy devices, a better understanding of how best to use older technologies is being obtained.
Phototherapy devices generate light by the conversion of electrical energy into electromagnetic energy. Filters and fluorophores are used to modify the output such that the desired wavelengths are emitted. There are several types of lamps (or bulbs) used to generate therapeutic UVR. These include incandescent lamps, arc lamps, and fluorescent lamps.
Incandescent lamps generate UVR by passing an electric current through a thin tungsten filament, which, in turn, generates heat and light. Because much of the electrical energy is converted to heat, these lamps are relatively inefficient light sources and have relatively short life spans. By sealing the tungsten filament in a quartz envelope that contains a halogen (bromine or iodine), the filament can be made to emit more energetic photons without reducing the longevity of the bulb. These lamps are called quartz halogen lamps and can emit wavelengths within the UV, visible, and IR ranges. In clinical dermatology, these lamps are employed primarily for situations such as phototesting and photodynamic therapy that require visible light.
Arc or gas discharge lamps were the first effective artificial sources of UVR. Arc lamps take advantage of the fact that when a high voltage is passed across two electrodes in the presence of a gas, the electrons of the gas atoms become excited. The arc of an arc lamp refers to the electric arc generated when the gas is ionized (ionized gas is also known as plasma) by a high electric current. When the gas electrons return to their ground state, light is emitted. The type of gas incorporated into the lamp determines the wavelengths that are emitted (i.e., spectral output). The output of arc lamps can be modulated by altering the gas pressure within the bulb such that at high pressures, the peak wavelength output broadens. High-pressure arc lamps typically contain mercury or xenon gas whereas low-pressure arc lamps utilize fluorescent material. In addition to altering the gas pressure to modify the spectral output of arc discharge lamps, the addition of metal halides broadens the output spectrum such that it becomes nearly continuous across the UV spectrum. For example, when mercury arc lamps are operated at high pressures, they have output emission peaks (so called mercury lines) seen at 297, 302, 313, 334, and 365 nm. In contrast, if a metal halide is added to the mercury, the output between these peaks is increased and is thus more continuous. The use of optical filters can then further refine the output of these lamps such that only the desired wavelengths are emitted. The advantage of metal halide lamps is the high output, which allows for shorter treatment times. However, they are more costly and more difficult to operate than fluorescent lamps. One example of a metal halide lamp currently in clinical use is the UVA1 light source.
Fluorescent lamps are the most commonly used sources of therapeutic UV light. These lamps take advantage of the fact that chemicals called phosphors (a specific type of chromophore also called a fluorophore) absorb and then reemit light. The light that is reemitted is of lower energy (and thus longer wavelength) than the inciting light. Using this principle, the UVC irradiation (that peaks at 254 nm) generated from a low-pressure mercury lamp can be converted to the longer UVB and UVA wavelengths of light that are desirable for phototherapy. The final output of a fluorescent lamp is dictated by the specific phosphor of the bulb. An important advance in photodermatology came with the development of a modified fluorescent lamp that emits largely at 311 nm.38 Broadband UVB and UVA light sources used for PUVA are other examples of fluorescent lamps.
Because different forms of phototherapy are used to treat different diseases, it is important and practical to divide devices based upon wavelength. Devices to deliver broadband UVB (BB-UVB), 311-nm narrowband UVB (NB-UVB), UVA (for use in psoralen photochemotherapy), and UVA1 (340–400 nm) are available in the United States and in most other countries. In addition to differing by spectral output, phototherapy devices range in the surface area that they are designed to treat (whole body, localized regions, or only lesional skin). Devices used for large body surface areas resemble booths (which patients enter for each treatment). These devices come in a variety of styles from round cylinders to folding units that can be unfolded for treatments and then collapsed while not in use. Devices have been developed to treat more limited areas (such as the palms and soles) and as such they are substantially smaller in size. Finally, targeted therapy utilizes devices that can deliver therapeutic ultraviolet radiation only to lesional skin and range in size from small handheld units to devices with a handheld wand attached to a larger UVR-generating component.
Originally used for psoriasis therapy, artificial sources of broadband UVB (BB-UVB) have been used therapeutically since the early twentieth century. In particular, UVB combined with the topical application of coal tar (as developed initially by William Goeckerman) was a mainstay of psoriasis treatment for many decades.39 More recently, with the development and availability of narrowband UVB, some dermatologists have concluded that BB-UVB is obsolete.40 However, BB-UVB is still widely used in the United States for a variety of conditions including psoriasis, atopic dermatitis, prurigo nodularis, and uremic pruritus.36 In addition, some patients who are unable to tolerate NB-UVB will respond to BB-UVB.36
The most commonly employed devices that deliver BB-UVB utilize fluorescent lamps. These devices emit UVR over a broad spectral range. Approximately, two-thirds of the output is in the UVB range and the rest is primarily in the UVA. However, because wavelengths within the UVB have nearly much more energy than wavelengths within the UVA, the UVA emitted contributes little to the therapeutic efficacy, assuming the patient is not taking a photosensitizing medication. BB-UVB and NB-UVB have been specifically designed to limit output below <290 nm (i.e., in the UVC range).
The wavelengths that most efficiently clear psoriasis are approximately 313 nm.41 In contrast, wavelengths less than 300 nm are the most efficient at causing erythema and nonmelanoma skin cancer. Based on this knowledge, light sources, termed narrowband UVB (NB-UVB), have been produced.38 These light sources emit only wavelengths between 308 and 313 nm, and have largely supplanted BB-UVB UV radiation sources for phototherapy. Although originally used to treat psoriasis, they have now been used to treat a number of other inflammatory skin diseases as well.
The initial starting dose of both broadband and narrowband UVB is determined in one of two ways (Tables 237-1 and 237-2). In the first, the minimal erythema dose (MED) is determined by exposing 6 one cm2 areas of skin on the inner aspect of the forearm or lower back to gradually increasing amounts of UV radiation from the same device that will be used for phototherapy. Twenty-four hours later, the UV exposed areas of skin are examined and the smallest UV dose that results in uniform erythema over the entire area, considered the MED, and phototherapy is initiated at 50% to 70% of that amount. Alternatively, the initial dose of phototherapy is established based empirically on Fitzpatrick skin phototype42 (see Tables 237-1 and 237-2). Subsequent exposures are given 2–5 times per week and the dose is increased at each treatment, assuming the patient has not developed an erythema response. If an erythema response has occurred, then, depending on its severity, the dose is either reduced or the treatment is delayed (see Tables 237-1 and 237-2). The maximum NB-UVB dose that should be administered is 2,000–5,000 mJ/cm2 depending on the photoreactive skin type. If patients miss treatments, dosage modifications should be made to avoid burns (see Table 237-5).
| ||
Fitzpatrick Skin Phototype | Initial NB-UVB Dose (mJ/cm2) | Maximum Dose (mJ/cm2) |
I | 130 | 2,000 |
II | 220 | 2,000 |
III | 260 | 3,000 |
IV | 330 | 3,000 |
V | 350 | 5,000 |
VI | 400 | 5,000 |
| |
Fitzpatrick Skin Phototype | Initial NB-UVB Dose (mJ/cm2) |
I | 20 |
II | 25 |
III | 30 |
IV | 40 |
V | 50 |
VI | 60 |
Psoralen-UVA photochemotherapy (known by the acronym PUVA) combines the oral ingestion or topical application of psoralens with exposure to UVR in the UVA range. Although psoralens and sunlight had been employed for thousands of years for the treatment of vitiligo, it was not until 1947 that PUVA in its modern form was described, initially for the treatment of vitiligo, and subsequently for the treatment of psoriasis.43
Three forms of psoralen are used in photochemotherapy regimens: 8-methoxypsoralen (8-MOP), 5-methoxypsoralen (5-MOP), and 4,5′,8-trimethylpsoralen (TMP). In the United States, only 8-methoxypsoralen is available. There are two oral formulations of 8-MOP, a micronized form which is typically given at a dose of 0.6 mg/kg 120 minutes prior to UVA exposure or a dissolved form which is given at a dose of 0.4–0.6 mg/kg 90 minutes before UVA exposure. The dissolved preparation is absorbed faster and yields higher and more reproducible serum levels and is therefore more commonly employed as a part of PUVA phototherapy regimens.
The most common sources of radiation for PUVA therapy are UVA fluorescent lamps, which have a maximum emission at 352 nm, near the absorption maximum for psoralens. For oral PUVA therapy, UVA radiation is usually initiated at a dose that corresponds to the 50%–70% of the minimum phototoxic dose (MPD) or according to the Fitzpatrick skin phototype (Table 237-3). The MPD is determined by having the patient take the dose of the oral psoralen to be used for the photochemotherapy treatment and exposing 6 one-cm2 areas of skin to gradually increasing doses of UVA. The MPD is evaluated 72 hours after UVA exposure and is the lowest amount of UVA that produces a uniform erythema over the entire area. In the United States, it is more common to initiate therapy based on the Fitzpatrick skin phototype (see Table 237-3). Treatments are usually given 2–4 times per week, avoiding consecutive days. The amount of UVA that is to be given is increased with each treatment. UVA dose modifications are made if an erythema response develops or treatments are missed (see Table 237-3).
| ||
| ||
Fitzpatrick Skin Phototype | Initial NB-UVB Dose (mJ/cm2) | Dose Increases (per week after the first week) |
I | 0.5 | 0.5 |
II | 1.0 | 0.5 |
III | 1.5 | 1.0 |
IV | 2.0 | 1.0 |
V | 2.5 | 1.5 |
VI | 3.0 | 1.5 |
Delivery of psoralens in bathwater is popular in some areas of the world because it provides a uniform drug distribution over the skin surface, is associated with very low psoralen plasma levels, and rapid elimination of free psoralens from the skin. This form of psoralen delivery circumvents gastrointestinal side effects and possible phototoxic hazards to the eyes associated with the oral form. Skin psoralen levels are highly reproducible, and photosensitivity lasts no more than two hours. Bath-PUVA consists of 15–20 minutes of whole-body immersion in solutions of 1-mg 8-MOP per liter of body temperature bathwater (Table 237-4). 5-MOP and trimethylpsoralen are also employed for bath PUVA. Irradiation is performed immediately after bathing, as photosensitivity decreases rapidly. Bath PUVA is started at 30% of the MPD. Treatments are typically given twice weekly. Guidelines for bath, local immersion, and other topical PUVA forms have been published by the British Photodermatology Group.44 A cost-effectiveness analysis of data collected across four centers in Scotland revealed that courses of both bath PUVA and other topical PUVA were consistently more expensive than oral PUVA.44 This is related predominantly to the increased nursing time required, although the cost of topical preparations also tends to be greater than oral preparations.
|
|
More recently, cream PUVA has been developed, which can be used to treat local and more widespread disease. Thirty minutes following the application of a psoralen-containing cream, patients are exposed to UVA irradiation.
Sunburn-like reactions are the most common short-term adverse effect of phototherapy. UVB burns usually peak at 12–24 hours, and PUVA burns at 24–48 or even 72 hours, with more severe burns peaking later than mild ones. Severe burns over a large portion of the skin surface produce systemic toxicity with fever and malaise in addition to pain. Severe PUVA burns, which extend well into the dermis, can lead to epidermal sloughing and are an indication for admission to a burn-care hospital facility. To avoid exacerbating a still developing PUVA burn, it is recommended that PUVA treatments not be given on consecutive days. Table 237-5 specifies the UVB or UVA dose adjustments in the event of a burn reaction during phototherapy. A burn reported by the patient at the next visit, even if no longer visible, should be managed in the same manner as a still-visible reaction. Burns over limited body areas, such as just the face or breasts, can be managed by local application of an appropriate sunscreen before or part way through subsequent treatments, especially if the area is not affected by the disease being treated. However, care must be taken to consistently protect the same area(s) in order to avoid a sudden full treatment to previously shielded skin.
No erythema | Increase by 25% |
Erythema with no pain | No increase |
Erythema with pain | Hold treatment until symptoms subside |
Erythema with pain and blistering | Hold treatment until symptoms subside and then reduce dose by 50% from last dose |
Modification of phototherapy dose for missed treatments: | |
<1 week | No increase in dose |
1–2 weeks | Decrease dose by 50% (BB-UVB) or 25% (NB-UVB or PUVA) |
2–3 weeks | Decrease dose by 75% (BB-UVB) or 50% (NB-UVB PUVA) |
>3 weeks | Restart at initial exposure dose |
Repeated UV-irradiated skin develops tolerance to subsequent exposures, allowing and indeed mandating progressively larger doses for optimal therapeutic effect. However, this tolerance is rapidly lost when exposures cease, requiring downward adjustments of dose after as little as 1 week (Table 237-5) to avoid burns.
Because of its longer wavelength, UVA1 phototherapy (340–400 nm) is able to penetrate more deeply into the skin than UVB or shorter range UVA called UVA2 (i.e., 320–340 nm). The first report describing a device capable of emitting UVA1 occurred in 1981.45 It was not until 1992 when the therapeutic benefit of UVA1 was demonstrated for atopic dermatitis that greater interest in the therapeutic properties of UVA1 occurred.46–47 Initially, one obstacle to the widespread use of the initial UVA1 devices was the intense heat that they generated. Newer UVA1 phototherapy units incorporating a specialized filtering and cooling system that removes nearly all wavelengths above 530 nm have largely eliminated this problem. Though not widely available in the United States, these light sources are useful for the management of a variety of dermatological conditions for which other forms of phototherapy are not helpful.48–49
UVA1 is administered 3–5 times per week. There have been several studies, which have attempted to establish the optimal amount of UVA1 to administer at each treatment session.50–54 Three dosing regimens have been used: (1) low dose (10–30 J/cm2), (2) medium dose (40–70 J/cm2), and (3) high dose (130 J/cm2).50–54 Although several comparison studies have been performed, at this point, there is no consensus as to which dose is best. In general, patients are started at 20–30 J/cm2 and increased to the full dose within 3–5 treatments.55 The risk of burns is far less than with UVB or PUVA therapy.
Unlike the previously described phototherapy devices that expose both lesional and uninvolved skin to UVR, targeted phototherapy delivers therapeutic doses of UVR only to lesional skin. Targeted phototherapy has also been called focused phototherapy, concentrated phototherapy, and microphototherapy. Several devices are available to deliver targeted phototherapy including both monochromatic (one wavelength) and polychromatic systems. There are several advantages to targeted phototherapy. These devices spare normal skin, thereby allowing higher fluences to be delivered to diseased skin while decreasing the risk of acute and chronic side effects to normal skin. Targeted therapy can be used on treatment-resistant lesions and on difficult anatomic locations (such as the scalp, chin, and nails). The handheld nature of a targeted phototherapy device may be easier for young children than receiving treatments in a phototherapy booth, which can be large and intimidating. The limitations of targeted phototherapy are device expense and the fact that it may not be practical for patients with more than 10% to 20% of body surface area involved to receive treatments in this manner. The preventive action of phototherapy on uninvolved but at-risk skin is also lost. Targeted phototherapy devices include excimer lasers and nonlaser devices known as monochromatic excimer light (MEL) devices.37 Both types of devices have been used to deliver targeted therapy to treat specific lesions of diseases such as psoriasis and vitiligo.36–37 While both types deliver monochromatic UVB irradiation (most commonly at 308 nm), they differ in several respects. Lasers typically deliver UVR to a smaller area but are capable of emitting higher amounts of radiation over a shorter period of time. In contrast, MEL devices deliver monochromatic irradiation to a larger area but with a lower power density. There are also several devices that emit polychromatic UVA or UVB (BB- or NB-UVB) to targeted areas. These devices typically utilize fiber-optic systems coupled with UVB generating sources. These devices have spot sizes from 1–3 cm. In addition, they have multiple delivery programs and automatic calibration, which makes treatment with predetermined dosages possible. These devices are smaller, less expensive, and have less maintenance problems than lasers.56–57 Treatment protocols with targeted phototherapy vary depending on the type of device that is employed.
Safety of Phototherapy
Safety principles are common to most phototherapy devices. Equipment should be checked on a regular basis by the clinical staff or the manufacturer’s engineer, since bulb output may change over time, and internal dosimetry components may fail. While phototherapy is usually delivered without incident, the risk of overtreatment is real, although the exact incidence of adverse events attributable to phototherapy is unknown and varies depending on the device. Importantly, with the exception of PUVA therapy for which formal long-term follow-up studies established an increased risk of lentigines, squamous cell carcinoma and melanoma, other forms of phototherapy appear to be remarkably safe.36,58 Newer therapies such as narrowband UVB and UVA1 appear to be relatively safe especially compared to nonphototherapeutic options for the same diseases, but await longer follow-up.
Repeated exposure of the skin to UV irradiation does result in cumulative actinic damage regardless of the source. With respect to nonmelanoma skin cancer, most studies have shown that there is little risk beyond that associated with habitual sun exposure with either BB-UVB or NB-UVB phototherapy.59–60 Greater than 300 treatments BB-UVB is associated with a modest but significant increase in SCC and BCC.61 However, the carcinogenic risk of a single PUVA treatment is about seven times greater than a single UVB treatment.62 As a result of its safety profile and efficacy, NB-UVB has emerged as a leading therapy for a number of skin diseases. Several studies have also shown that long-term exposure to BB-UVB combined with topical tar preparations is not associated with an increased risk of SCC.63
The side effects of PUVA include drug intolerance reactions as well as the combined action of psoralens plus UVA radiation. Oral 8-MOP may cause nausea (10% of patients) and vomiting, and this occasionally necessitates discontinuation of treatment. These side effects are more common with liquid preparations rather than with crystalline preparations, probably because of higher psoralen serum levels. The nausea may be minimized or avoided by instructing the patient to take 8-MOP with milk, food, or ginger (e.g., ginger cookies, ginger ale, ginger supplements)64 or to divide the dose into two portions, taken approximately one-half hour apart. Other reported effects include nervousness, insomnia, and depression. With 5-MOP, nausea is rare, even with doses up to 1.8 mg/kg body weight.
Following exposure to UVA, approximately 10% of patients undergoing PUVA therapy will experience pruritus. In most cases, this can be alleviated by bland emollients. Some patients with severe pruritus may require systemic treatment. A stinging pain may rarely occur, and the mechanism for this is unknown. The symptoms are usually unresponsive to antihistamines, and, in most instances, subside when the treatment is discontinued.
Mild and often transient focal erythema after PUVA therapy occurs frequently. Any area showing erythema with tenderness or blistering should be shielded during subsequent UVA exposures until the erythema has resolved. As noted above, erythema appearing within 24 hours may signal a potentially severe phototoxic reaction, and may worsen progressively over the next 24 hours, since peak erythema with PUVA characteristically occurs at least 48 hours after the treatment. In that situation, patients should be protected from further UVA exposures and sunlight, and should be monitored closely until the erythema has resolved.
Very rare side effects of PUVA include polymorphous light eruption-like rashes, acne-like eruptions, subungual hemorrhages caused by phototoxic reactions of the nail beds, onycholysis of the nails, and occasionally hypertrichosis of the face. These disappear when treatment is discontinued. Analysis of laboratory data in several large studies revealed no significant abnormal findings in patients receiving PUVA over prolonged periods of time.65–67
Chronic exposure to PUVA may result in skin changes that resemble photoaging, and which is aggravated by chronic natural sun exposure. PUVA lentigines are small brown macules with irregular borders and uneven pigmentation68 and are histologically characterized by proliferation of large melanocytes.69 In contrast to solar lentigines, melanocytes in PUVA lentigines often display an increased size of melanosomes, clustering and binucleation with nuclear hyperchromatism, and cellular pleomorphism. T1799A B-type Raf (BRAF) mutations have been found to be present in PUVA lentigines,70 but the full significance of this is not yet understood, as both cutaneous malignant melanoma and benign melanocytic nevi often have BRAF mutations.71–74 The presence of these lesions is directly related to the number of PUVA treatments and total UVA dose that has been administered. The absence of PUVA lentigines serves as a useful indicator of a lower risk of PUVA malignancy.75
Cutaneous malignancies are the major concern of long-term and repeated PUVA treatments. The risk of nonmelanoma skin cancer and possibly malignant melanoma increases in a dose-dependent manner. In laboratory animals, 8-MOP and 5-MOP have unequivocally induced skin cancer at levels of drug and UVA irradiation comparable to those used in PUVA therapy.76 Cancer development is thought to stem from both DNA damage and down-regulation of the immune system. The PUVA Follow-up Study, which evaluated 1,380 patients who began PUVA treatment for psoriasis in 1975 and 1976 has documented major health events in these individuals in a prospective manner. Overall, patients who were treated with at least 337 PUVA treatments exhibited a 100-fold increased risk of SCC compared to that expected from population incidence rates.77 Moreover, almost 4% of patients with SCC developed metastases, most commonly originating in the genital area. There is uncertainty about PUVA being the sole factor as many of the patients in the long-term follow-up studies also had significant exposure to sunlight and to treatments with carcinogenic potential, including arsenic, UVB, and methotrexate. The risk of developing SCC with PUVA may be further potentiated by the use of cyclosporine and, for this reason, cyclosporine is contraindicated in individuals who have been treated with PUVA.78 Oral retinoids used concurrently with PUVA, on the other hand, reduce the risk of SCC.79
Individuals treated with PUVA are at increased risk of cutaneous malignancies of the genitalia, and this has led to standard protection of the genitalia during phototherapy.80 The risk is dose-dependent, with a 90-fold increased risk of genital tumors among patients exposed to high doses of PUVA compared with that expected in the general population. Men treated with high-dose exposures to both PUVA and topical tar/UVB have the greatest risk of genital tumors.80–81 There is currently no standardized regimen for genital shielding. There are pouches for genital shielding that are commercially available, but the cost and availability may be prohibitive. Factors influencing the effectiveness of UV irradiation protection include fiber composition, porosity (intrinsic to which is number of layers, weave type, and thread count), mass, and color.82–83 Commonly used protective agents include surgical masks, paper towels, blue surgical towels, and underwear. The efficacy of these materials has been studied, and surgical masks were found to provide insufficient protection against UV irradiation most likely due to increased porosity (looser weave) and decreased mass.84
The relationship between PUVA and melanoma has also been examined in detail. The PUVA Follow-Up Study has provided evidence that individuals with at least 250 treatments and at least 15 years from the first PUVA treatment were at increased risk of developing melanoma.85 Patients who developed a phototoxic reaction more easily were at higher risk for melanoma than those with darker skin.86 As a result of those studies, a personal or family history of melanoma or a history of greater than 200 PUVA treatments is considered to be a relative contraindication to further PUVA therapy.86
In patients employing PUVA therapy in combination with methotrexate for at least 36 months, the incidence of lymphoma was more than 7 times higher than that of cohort members earlier in the study who had not taken methotrexate.87
UVA is absorbed in the lens and in the presence of UVA, psoralens can bind protein, DNA, and RNA. Because the lens never sheds its cells, protein-bound 8-methoxypsoralen accumulates in the lens, increasing the risk of irreversible opacification.88 There have been reports of various ocular problems in patients on PUVA including cataracts, 89–90 conjunctival hyperemia,91 and decreased lacrimation.91
A 25-year prospective study92 sought to evaluate the effect of PUVA on the eyes. Participants were instructed to use UVA-blocking eyewear when outside or looking outside through window glass during daylight for a minimum of 12 hours, although current labeling calls for 24 hour protection. This study found no relationship between increasing numbers of PUVA sessions and visual impairment or cataracts, and demonstrates that increasing exposure to PUVA does not increase cataract risk among middle-aged and older persons using eye protection as practiced by this cohort.92 Other smaller studies have also found no increase in cataract formation or visual impairment.93–96
UVA1 phototherapy is generally well tolerated.52–53 Reported side effects include intense tanning, erythema, pruritus, urticaria, tenderness, a burning sensation, polymorphous light eruption, eczema herpeticum and bacterial superinfection.48–49,54 However, because UVA1 phototherapy has only been available since the 1990s, the long-term effects are still under investigation.
The safety of phototherapy and photochemotherapy in HIV-positive patients has been debated. Ultraviolet radiation may activate HIV by the induction of NF-κB,97–99 and UVB therapy increases HIV-1 gene expression in the skin.100–102 However, BB-UVB phototherapy does not appear to affect plasma HIV levels nor does it have an effect on CD4 counts.101,103–104 In general, phototherapy is thought to be safe for HIV patients.105 A consensus statement published by the American Academy of Dermatology in 2010 concluded that for moderate-to-severe psoriasis in HIV-positive patients, phototherapy and antiretrovirals are the recommended first-line therapeutic agents.106
NB-UVB is now preferred to PUVA in children for most skin conditions, because of concern about PUVA side effects including phototoxicity, carcinogenicity, photoaging, and the potential development of cataracts.
Patients who have had arsenic exposure are at increased risk for cutaneous malignancies and should avoid phototherapy. Transplant patients have a much higher risk of skin cancer compared to the general population and, in that patient population there is a relative contraindication to phototherapy. Photosensitizing medications should theoretically be avoided during phototherapy treatment, although in practice many patients uneventfully receive phototherapy while taking tetracycline, hydrochlorothiazide, or other photosensitizing drugs. There are anecdotal reports of an association between chronic use of voriconazole and the development of aggressive cutaneous malignancies including melanoma.107–110
Diseases Amenable to Phototherapy
Responses of the diseases listed in Table 237-6 are discussed in detail online. See also Chapter 238.
NB-UVB/BB-UVB | PUVA | UVA1 | |
|---|---|---|---|
Psoriasis | + | + | − |
Atopic dermatitis | + | + | + (acute flares) |
Cutaneous T-cell lymphoma | + | + | + |
Vitiligo | NB-UVB | + | − |
Photodermatoses | +/− | + | − |
Pruritus of chronic renal failure | + | − | − |
Cholestatic pruritus | + | − | − |
Aquagenic pruritus | − | + | − |
Pruritus of polycythemia vera | + | + | − |
Localized and systemic scleroderma | − | + | + |
Chronic GVHD | + | + | + |
Pityriasis lichenoides | + | + | − |
Lymphomatoid Papulosis | + | − | + |
Telangiectasis macularis eruptiva Perstans | − | + | + |
Urticaria pigmentosa | − | +/− | + |
Granuloma annulare | − | + | + |
Lichen planus | + | + | − |
Chronic hand eczema | + | + | + |
Perforating disorders | + | + | − |
![]() Both BB-UVB and NB-UVB have been employed to treat psoriasis. Their presumptive mechanisms of action in this disease include its effects on DNA, antimicrobial actions which may alter the microbial flora of the skin,111 induction of anti-inflammatory and immunosuppressive cytokines,112–114 augmentation of vitamin D levels,115–116 alterations in antimicrobial peptides,116 and a reduction in C-reactive protein.117
Both BB-UVB and NB-UVB have been employed to treat psoriasis. Their presumptive mechanisms of action in this disease include its effects on DNA, antimicrobial actions which may alter the microbial flora of the skin,111 induction of anti-inflammatory and immunosuppressive cytokines,112–114 augmentation of vitamin D levels,115–116 alterations in antimicrobial peptides,116 and a reduction in C-reactive protein.117
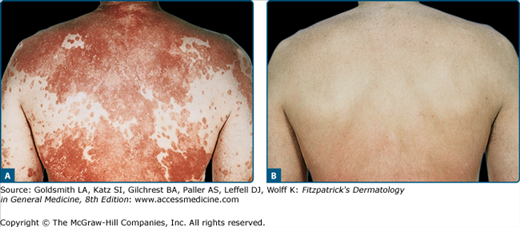
![]() Clinical trials38 supporting the research findings41 that the wavelengths most effective at clearing psoriasis are in the range of 313 nm have led to broad utilization of NB-UVB for psoriasis (eFig. 237-0.1). NB-UVB has thus become first-line therapy for chronic plaque psoriasis, considered to be superior to conventional broadband UVB with respect to both clearing and remission times.38,118 The difference in efficacy may be related to more efficient clearing of T cells by NB-UVB from the epidermis and dermis of psoriatic plaques compared with conventional broadband UVB.20 The endpoint of phototherapy is complete clearance of all psoriatic skin lesions. Psoriasis, however, is a chronic disease and the remission induced by UVB phototherapy is often short lived. In a randomized, prospective, multicentered trial, investigators found that continuing UVB phototherapy after initial clearing contributes to the control of the disease and is justified for many patients.119 In such maintenance therapy, the frequency of UVB treatments is reduced while maintaining the last dose given at the time of clearing. NB-UVB can be used safely in pregnancy and in children.
Clinical trials38 supporting the research findings41 that the wavelengths most effective at clearing psoriasis are in the range of 313 nm have led to broad utilization of NB-UVB for psoriasis (eFig. 237-0.1). NB-UVB has thus become first-line therapy for chronic plaque psoriasis, considered to be superior to conventional broadband UVB with respect to both clearing and remission times.38,118 The difference in efficacy may be related to more efficient clearing of T cells by NB-UVB from the epidermis and dermis of psoriatic plaques compared with conventional broadband UVB.20 The endpoint of phototherapy is complete clearance of all psoriatic skin lesions. Psoriasis, however, is a chronic disease and the remission induced by UVB phototherapy is often short lived. In a randomized, prospective, multicentered trial, investigators found that continuing UVB phototherapy after initial clearing contributes to the control of the disease and is justified for many patients.119 In such maintenance therapy, the frequency of UVB treatments is reduced while maintaining the last dose given at the time of clearing. NB-UVB can be used safely in pregnancy and in children.
![]() Other agents (topical and oral) combined with UVB phototherapy may clear psoriatic skin lesions in a shorter period of time. Combined therapies may thus allow for fewer treatments and potentially less photoaging and other risks. Often combination regimens are administered when phototherapy alone is no longer effective. Oral retinoids, such as acitretin,120 increase efficacy, particularly in patients with chronic plaque type psoriasis.121–123 Although fewer sessions may be required with this combination therapy, the rate of relapse may be slightly higher. Methotrexate (15 mg/week) administered 3 weeks before starting NB-UVB can also allow for quicker results in fewer phototherapy sessions.124 More recently, biologic therapies have been evaluated in conjunction with phototherapy. Treatment with NB-UVB significantly accelerated the clearance of psoriatic lesions in patients responding slowly to etanercept.125 Other studies have evaluated alefacept in combination with NB-UVB and have shown improved and more rapid clearance of psoriatic lesions.126
Other agents (topical and oral) combined with UVB phototherapy may clear psoriatic skin lesions in a shorter period of time. Combined therapies may thus allow for fewer treatments and potentially less photoaging and other risks. Often combination regimens are administered when phototherapy alone is no longer effective. Oral retinoids, such as acitretin,120 increase efficacy, particularly in patients with chronic plaque type psoriasis.121–123 Although fewer sessions may be required with this combination therapy, the rate of relapse may be slightly higher. Methotrexate (15 mg/week) administered 3 weeks before starting NB-UVB can also allow for quicker results in fewer phototherapy sessions.124 More recently, biologic therapies have been evaluated in conjunction with phototherapy. Treatment with NB-UVB significantly accelerated the clearance of psoriatic lesions in patients responding slowly to etanercept.125 Other studies have evaluated alefacept in combination with NB-UVB and have shown improved and more rapid clearance of psoriatic lesions.126
![]() Phototherapy is commonly combined with topical agents in order to achieve higher clearance rates, longer disease-free intervals, and a lower risk of side effects. The Goeckerman regimen consists of the application of tar-containing topical agents with subsequent UV irradiation. The use of liquid carbonis detergens with NB-UVB is one example of a modified form of this regimen. The regimen has been shown to be safe, convenient, effective, and leads to more rapid improvement of psoriasis than light therapy alone.127 The antipsoriatic vitamin D analogs calcipotriol and calcitriol, used in conjunction with NB-UVB, provide additional benefit compared to either the drugs or the phototherapy alone.128–133 A combination of calcipotriol with phototherapy leads to degradation of vitamin D3, and calcipotriene has been shown to increase the minimal erythema dose in patients, suggesting that it has a photoprotective effect.134 For these reasons, when used in combination with phototherapy, vitamin D analogs are applied after the light treatment. Tazarotene 0.1% gel has an additive or synergistic effect when combined with NB-UVB.135 Because retinoids can cause photosensitivity, it is common practice to initiate phototherapy at somewhat lower doses when tazarotene is used with UVB.136 Topical bexarotene gel 1% has also been combined with NB-UVB for the treatment of psoriasis and been shown to have greater efficacy than either alone.137 By decreasing scatter from scales in the stratum corneum, lubricants improve transmission of UVB, and a combination of topical lubricants with UVB therapy increases efficacy.138–140 The use of topical salicylic acid with UVB has not been found to increase the efficacy of UVB phototherapy because it blocks UVB penetration.
Phototherapy is commonly combined with topical agents in order to achieve higher clearance rates, longer disease-free intervals, and a lower risk of side effects. The Goeckerman regimen consists of the application of tar-containing topical agents with subsequent UV irradiation. The use of liquid carbonis detergens with NB-UVB is one example of a modified form of this regimen. The regimen has been shown to be safe, convenient, effective, and leads to more rapid improvement of psoriasis than light therapy alone.127 The antipsoriatic vitamin D analogs calcipotriol and calcitriol, used in conjunction with NB-UVB, provide additional benefit compared to either the drugs or the phototherapy alone.128–133 A combination of calcipotriol with phototherapy leads to degradation of vitamin D3, and calcipotriene has been shown to increase the minimal erythema dose in patients, suggesting that it has a photoprotective effect.134 For these reasons, when used in combination with phototherapy, vitamin D analogs are applied after the light treatment. Tazarotene 0.1% gel has an additive or synergistic effect when combined with NB-UVB.135 Because retinoids can cause photosensitivity, it is common practice to initiate phototherapy at somewhat lower doses when tazarotene is used with UVB.136 Topical bexarotene gel 1% has also been combined with NB-UVB for the treatment of psoriasis and been shown to have greater efficacy than either alone.137 By decreasing scatter from scales in the stratum corneum, lubricants improve transmission of UVB, and a combination of topical lubricants with UVB therapy increases efficacy.138–140 The use of topical salicylic acid with UVB has not been found to increase the efficacy of UVB phototherapy because it blocks UVB penetration.
![]() Targeted phototherapy using a monochromatic 308-nm excimer laser or monochromatic excimer light is effective and safe for psoriasis. A large, multicenter study found that fewer patient visits were required with targeted phototherapy compared to conventional phototherapy.141 The use of monochromatic excimer compared to cream PUVA has been found to have equivalent efficacy for palmoplantar psoriasis.142
Targeted phototherapy using a monochromatic 308-nm excimer laser or monochromatic excimer light is effective and safe for psoriasis. A large, multicenter study found that fewer patient visits were required with targeted phototherapy compared to conventional phototherapy.141 The use of monochromatic excimer compared to cream PUVA has been found to have equivalent efficacy for palmoplantar psoriasis.142
![]() Oral PUVA has been shown to consistently induce remission of psoriasis in clinical studies.65,143,144 At least 75% improvement in PASI score can be expected after 12 weeks of PUVA treatments in 60% of patients.143 In some studies, oral PUVA has been observed to be more efficacious in clearing plaque psoriasis than NB-UVB, and the duration of remissions is more prolonged,144 while other studies have found the two treatments to be comparable, particularly when NB-UVB is used three times a week.145–148 It must be noted here that unlike drug administration, the success of PUVA and phototherapy generally depends in large part on physician-determined patient-specific subtle modifications of the regimen, such as the dose increments between treatments, that optimize therapeutic response while avoiding burns or other adverse effects. Hence, the response to phototherapy may vary greatly among studies and among practitioners.
Oral PUVA has been shown to consistently induce remission of psoriasis in clinical studies.65,143,144 At least 75% improvement in PASI score can be expected after 12 weeks of PUVA treatments in 60% of patients.143 In some studies, oral PUVA has been observed to be more efficacious in clearing plaque psoriasis than NB-UVB, and the duration of remissions is more prolonged,144 while other studies have found the two treatments to be comparable, particularly when NB-UVB is used three times a week.145–148 It must be noted here that unlike drug administration, the success of PUVA and phototherapy generally depends in large part on physician-determined patient-specific subtle modifications of the regimen, such as the dose increments between treatments, that optimize therapeutic response while avoiding burns or other adverse effects. Hence, the response to phototherapy may vary greatly among studies and among practitioners.
![]() Repeated exposures are required to clear PUVA-responsive diseases, with gradual dose increments as pigmentation develops. Upon clearing, patients are often transitioned to maintenance therapy, during which the frequency of treatments is gradually reduced. There are various maintenance algorithms. One regimen consists of 1 month of twice-weekly treatments, at the last UVA dose used for clearing, followed by another month of once-weekly exposures. Other protocols recommend maintenance treatment only when the patient relapses rapidly. This has the advantage of avoiding higher cumulative exposure and thus greater risk of long-term side effects.
Repeated exposures are required to clear PUVA-responsive diseases, with gradual dose increments as pigmentation develops. Upon clearing, patients are often transitioned to maintenance therapy, during which the frequency of treatments is gradually reduced. There are various maintenance algorithms. One regimen consists of 1 month of twice-weekly treatments, at the last UVA dose used for clearing, followed by another month of once-weekly exposures. Other protocols recommend maintenance treatment only when the patient relapses rapidly. This has the advantage of avoiding higher cumulative exposure and thus greater risk of long-term side effects.
![]() A combination of PUVA and methotrexate can reduce the duration of treatment, number of exposures, and total UVA dose required for clearing and is also effective in clearing patients unresponsive to PUVA alone.149
A combination of PUVA and methotrexate can reduce the duration of treatment, number of exposures, and total UVA dose required for clearing and is also effective in clearing patients unresponsive to PUVA alone.149
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree