Photochemotherapy and Photodynamic Therapy: Introduction
|
Photochemotherapy
Photochemotherapy with psoralens combines the use of oral or topical psoralens (P) and ultraviolet A radiation (UVA), termed PUVA. Psoralens are phototoxic compounds that enter cells and then absorb photons to produce photochemical reactions that alter the function of cellular constituents.1 This interaction results in a beneficial therapeutic effect after repeated controlled phototoxic reactions. Psoralens can be administered orally or applied topically to the skin in the form of solutions, creams, or baths. This therapy is currently used in the treatment of several common and uncommon skin diseases.
In the 1970s, it was shown that orally administered 8-methoxypsoralen (8-MOP) and subsequent irradiation with artificial UVA was a highly effective treatment for psoriasis.2,3 Psoralen baths (soaking in a dilute psoralen solution) and subsequent UVA exposure (bath-PUVA), which originated in Scandinavia,4 is also being used in many European institutions. The effectiveness of all variants of PUVA has been widely confirmed and has profoundly influenced dermatologic therapy, in general, providing treatment for numerous disorders in addition to psoriasis (Table 238-1). A major advance in phototherapy was the development of fluorescent bulbs that emitted narrowband UVB radiation at 311–313 nm in the mid-1980s. This narrow spectrum is slightly inferior in clearing psoriasis or mycosis fungoides. However, due to the fact that it is easier to perform and possibly safer than PUVA, it is now more frequently used in many phototherapy centers. Narrowband UVB phototherapy is also beneficial for a variety of other dermatoses that were previously treated with PUVA. Nevertheless PUVA has still remained the mainstay for recalcitrant diseases.
Therapy of Disease | Prevention of Disease Symptoms |
|---|---|
|
|
The rationale for PUVA therapy is to induce remissions of skin diseases by repeated, controlled phototoxic reactions. These reactions occur only when psoralens are photoactivated by UVA. Due to the penetration characteristics of UVA, absorption of photons is confined to the skin. However, there is also some evidence that PUVA may exert systemic effects through circulating lymphocytes affected while transiting through the skin. Clinically, PUVA-induced phototoxic reactions are characterized by a delayed sunburn-like erythema and inflammation.
Three psoralens are used in PUVA therapy. Methoxsalen or 8-methoxysporalen (8-MOP), obtained from the seeds of a plant called Ammi majus, is most widely used and the only psoralen available in the United States. Bergapten or 5-methoxypsoralen (5-MOP) and trioxsalen or 4,5′,8-trimethylpsoralen (TMP) are available in Europe and elsewhere.
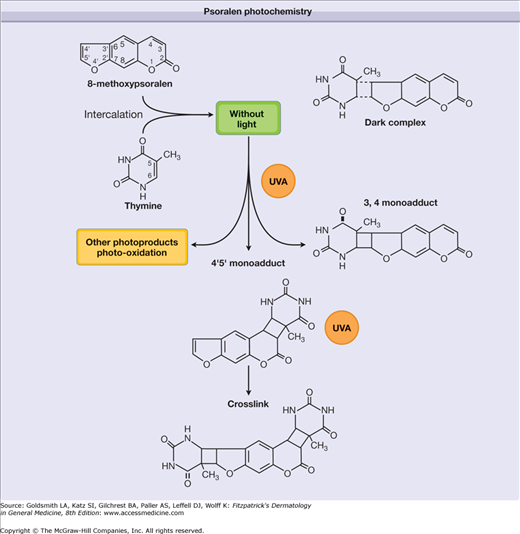
Psoralens intercalate between apposing DNA base pairs in the double helix in the absence of UV radiation. Absorption of photons in the UVA range results in the formation of a 3,4- or 4,5-cyclobutane addition product (adduct) with pyrimidine bases of native DNA. In the first step of this photochemical reaction, a monofunctional adduct with thymine or cytosine is formed. Some psoralens, including 8-MOP, TMP, and 5-MOP, can absorb a second photon, and this reaction leads to the formation of a bifunctional adduct with a 5,6 double bond of the pyrimidine base of the opposite strand, thus producing an interstrand cross-link of the double helix (Fig. 238-1). This intercalation of psoralens with epidermal DNA suppresses both DNA synthesis and cell division, and it was originally assumed that this was the therapeutic mechanism in psoriasis. However, cross-linking does not appear to be a prerequisite for the therapeutic effect,7 and successful PUVA treatment of other skin diseases is unlikely to be directly caused by this molecular reaction; psoralens also react with RNA, proteins, and other cellular components, and indirectly modify proteins and lipids via singlet oxygen-mediated reactions or by generating free radicals.7 Perhaps these mechanisms contribute to the effects of PUVA in diseases that are not hyperproliferative in nature.
Figure 238-1
Psoralen photochemistry. Psoralens intercalate between apposing DNA base pairs forming a “dark complex.” Absorption of ultraviolet A (UVA) photons leads to a 3,4- or 4,5-cyclobutane monoadduct with pyrimidine bases of native DNA. The absorption of a second photon results in a bifunctional adduct producing an interstrand cross-link of the double helix.
The formation of mono- and bifunctional photoadducts in DNA results in the immediate inhibition of DNA synthesis. The interstrand cross-links are believed to be largely responsible for eliciting skin photosensitization reactions of linear psoralens such as 8-MOP. Excessive production of these cyclobutane adducts causes cell death. Mutation and skin carcinogenesis also result from photoconjugation of psoralens to DNA because the cells surviving this DNA damage tend to repair it through an error-prone repair process.7
In type II reactions (see Chapter 90), reactive oxygen species (1O2, O2, or OH) induce the oxidation of cellular lipoprotein membrane lipids and destruction of membrane-bound cytochrome P450. The membrane-damaging events activate the arachidonic acid metabolism pathway, which results in an increase of secondary oxidation products that contribute to the increased synthesis of eicosanoids. Furthermore, the reactive oxygen species can directly damage DNA by generating DNA strand breaks.7
Hypotheses about the mechanism of action in psoriasis are based on the known photoconjugation of psoralens to DNA with subsequent suppression of mitosis, DNA synthesis, and cell proliferation, expected to revert increased cell proliferation rates in psoriasis to normal. However, PUVA also alters the expression of cytokines and cytokine receptors, downregulates certain lymphocyte and antigen-presenting cell functions, influences adhesion molecule expression, and diminishes Langerhans cell numbers within the epidermis. In addition, PUVA affects immune effector cells such as lymphocytes or polymorphonuclear leukocytes. Because there is evidence that psoriasis is caused primarily by the action of blood-derived immunocytes, it is reasonable to speculate that PUVA therapy may act by affecting immune function through a direct phototoxic effect on lymphocytes in skin infiltrates. This is consistent with the observation that several other disorders that are not hyperproliferative in nature but immunomediated also respond well to PUVA. PUVA can revert pathologically altered patterns of keratinocyte differentiation and reduce the number of proliferating epidermal cells. Infiltrating lymphocytes are strongly suppressed by PUVA, with variable effects on different T-cell subsets. Lymphocytes are far more likely to undergo apoptosis than keratinocytes8 in response to PUVA, which may explain the high efficacy in cutaneous T-cell lymphoma (CTCL), as well as in inflammatory skin diseases including psoriasis that is now recognized to be in part T-cell mediated as well as hyperproliferative. Although much is known about pathways and mechanisms of psoralen photosensitization, the interactions and relative contributions to the clearing of a specific disease are not well understood.
Psoralens also stimulate melanogenesis. This involves the photoconjugation of psoralens to DNA in melanocytes, mitosis, and subsequent proliferation of melanocytes, an increased formation and melanization of melanosomes, an increased transfer of melanosomes to keratinocytes, and activation and increased synthesis of tyrosine mediated in part by stimulation of cAMP activity.
The important steps between the ingestion of a psoralen and its arrival at the site of action include absorption, first-pass effect, blood transportation, and tissue distribution. The absorption rate of a psoralen from the gut depends mainly on the physical characteristics of the preparation and the fat content of the concomitant food intake. Liquid preparations of 8-MOP and 5-MOP give higher and earlier peak serum levels than do crystalline formulations. In addition, peak serum levels are achieved by liquid preparations after a relatively reproducible time interval in all subjects, whereas wide time variability occurs with crystalline formulations. Before reaching the skin via the circulation, psoralens are metabolized during passage through the liver. Plasma levels of 8-MOP administered orally at different doses show a strong nonlinearity, indicating a saturable first-pass effect. The unpredictable pharmacokinetic behavior is probably due to inter- and intraindividual variations of intestinal absorption, first-pass effect, blood distribution in the body, metabolism, and elimination of the drug.
Within the same individual, serum levels of 8-MOP correspond fairly well with skin reactivity, the peak of skin phototoxicity coinciding with peak serum levels. However, phototoxic responses to PUVA show large interindividual variations. Hence, measurement of serum psoralens is a research tool and not used to monitor clinical therapy.
The pharmacokinetics of 8-MOP after topical treatment depend on the method of application. 8-MOP topically applied as a 0.15% emulsion or solution leads to plasma levels comparable to those found with oral treatment if large areas of the body are treated. In contrast, plasma levels after bath-PUVA treatment of almost the total body surface are very low. Bathwater-delivered psoralens are readily absorbed in the skin but are promptly eliminated without cutaneous accumulation.8
UVA sources commonly used for PUVA therapy are fluorescent lamps or high-pressure metalhalide lamps. The typical fluorescent PUVA lamp has an emission peak at 352 nm and emits approximately 0.5% in the UVB range. UVA doses are given in Joules per centimeter2, usually measured with a photometer with a maximum sensitivity at 350–360 nm. Although the action spectrum of antipsoriatic activity and phototoxic erythema peaks at 335 nm, longer wavelengths have proved equally effective for clearing psoriasis if delivered in an adequate dose to obtain an equal erythemogenic response.9
PUVA treatment produces an inflammatory response that manifests as delayed phototoxic erythema, proportional to the dose of both drug and UVA as well as to the individual’s sensitivity to phototoxic reactions. 8-MOP dose changes within individuals, over a narrow but clinically relevant range, appears to significantly alter the threshold for PUVA erythema, but not the rate of increase in erythema intensity with increasing UVA dose.10 Importantly, the time course of PUVA erythema differs from sunburn or UVB erythema that appears after 4–6 hours and peaks 12–24 hours after exposure. PUVA erythema does not appear before 24–36 hours and peaks at 72–96 hours, or even later. Hence, daily PUVA treatments can result in unexpected severe delayed cumulative phototoxicity. PUVA erythema has a shallower dose-response curve than UVB erythema (by a factor of approximately 2) and this difference is maintained even at the point of maximum erythema.11 Severe PUVA reactions may lead to blistering and to superficial skin necrosis. Overdoses of UVA are frequently followed by swelling, intense pruritus, and, sometimes, by a stinging sensation in the affected skin area, possibly as a consequence of damage of superficial nerve endings. Erythema is at present the only available parameter that allows an assessment of the magnitude of the PUVA reaction; thus, it represents an important criterion for dose adjustments.9
Pigmentation is the second important effect of PUVA. It may develop without clinically evident erythema, especially when oral 5-MOP or TMP is used; this is particularly important in the treatment of vitiligo and for the preventive therapy of certain photodermatoses. In unaffected skin, PUVA pigmentation is maximal approximately 7 days after a PUVA exposure and may last from several weeks to months. As with sun-induced pigmentation, the individual’s ability to tan is genetically determined, but the dose-response curve is much steeper. A few PUVA exposures result in a much deeper tan than that produced by multiple exposures to solar radiation.
Application of 8-MOP in creams, ointments, or lotions followed by UVA irradiation is effective in clearing psoriasis but has several disadvantages. The nonuniform distribution on the skin surface induces unpredictable phototoxic erythema reactions and irregular patches of cosmetically unacceptable hyperpigmentation. Furthermore, if numerous lesions are present, the application is laborious and time consuming, and the treatment does not prevent the development of new active lesions in previously unaffected, untreated areas. Therefore, topical PUVA with psoralen creams, ointments, or lotions is now used only for limited plaque psoriasis and for palmoplantar disease.
The use of bathwater delivery of psoralens provides for a uniform drug distribution over the skin surface, very low psoralen plasma levels, and rapid elimination of free psoralens from the skin. Bathwater delivery of 8-MOP circumvents gastrointestinal side effects and possible phototoxic hazards to the eyes because there is no systemic photosensitization. Skin psoralen levels are highly reproducible, and photosensitivity lasts no more than 2 hours. The higher incidence of unwanted burn reactions can be prevented by a lower starting dose [50% of the minimal phototoxic dose (MPD)] and a more cautious dosimetry in the initial treatment phase. A major drawback in many treatment facilities is the requirement for a bathtub. Originally, bath-PUVA was performed with TMP, but 8-MOP and 5-MOP are now being used as well. Bath-PUVA consists of 15–20 minutes of whole-body immersion in solutions of 0.5- to 5.0-mg 8-MOP per liter of bathwater. Irradiation needs to be performed immediately, as photosensitivity decreases rather rapidly. TMP is more phototoxic after topical application and is used at lower concentrations than 8-MOP. Minimal phototoxicity dose (MPD) determination for bath-PUVA must take into account that the phototoxic threshold declines during the early treatment phase,8 in contrast to oral PUVA. Guidelines for bath, local immersion, and other topical PUVA forms have been published by the British Photodermatology Group and are based, where possible, on the results of controlled studies, or otherwise on consensus.12
In oral PUVA, 8-MOP is administered orally (0.6–0.8 mg/kg body weight) 1–3 hours before exposure, depending on the absorption characteristics of the particular drug brand. Liquid drug preparations are absorbed faster and yield higher and more reproducible serum levels than microcrystalline forms. For 5-MOP, the usual dosage is 1.2- to 1.8-mg/kg body weight.
The initial UVA doses are determined by either the patient’s skin type13–15 or by MPD testing.9 The MPD is defined as the minimal dose of UVA that produces a barely perceptible, but well-defined, erythema when template areas of the skin are exposed to increasing doses of UVA. Erythema readings are performed 72 hours after testing, at which time the psoralen phototoxicity reaction usually reaches its peak. The MPD test should be performed on previously nonexposed skin (e.g., buttocks). Although the MPD test is more time-consuming than phototyping, it allows for more accurate and higher UVA doses during initial treatment. Table 238-2 shows recommendations for dosimetry in bath and oral PUVA.
Determination of MPD | Reading | After 96–120 hours (Bath-PUVA) After 72–96 hours (Oral PUVA) |
|---|---|---|
Start of treatment | First therapeutic dose | 30% of MPD (bath-PUVA) 50%–70% of MPD (oral PUVA) |
Treatments two to four times weekly | No erythema, good response No erythema, no response Minimal erythema Persistent asymptomatic erythema Painful erythema (with or without blistering) | Increase once weekly by 30% Increase by 30% per session No increase No increase No treatment until symptoms subside |
Resumption of treatment after missed sessions | After resolution of symptoms | Reduction of last dose by 50%; next increase by 10% |
Repeated exposures are required to clear PUVA-responsive diseases, with gradual dose increments as pigmentation develops. Lower doses quite frequently result in failure of treatment except in those diseases in which induction of pigmentation is the desired objective. In most dermatoses amenable to PUVA, the frequency of treatments is reduced after satisfactory clearing of disease, and the last UVA dose is used as a maintenance dose, if maintenance treatment is planned. The duration of this maintenance phase and the frequency of treatments depend on the particular disease being treated and its propensity to relapse.
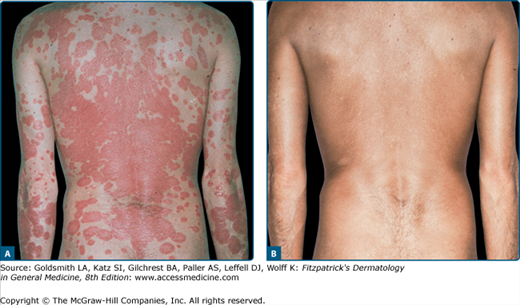
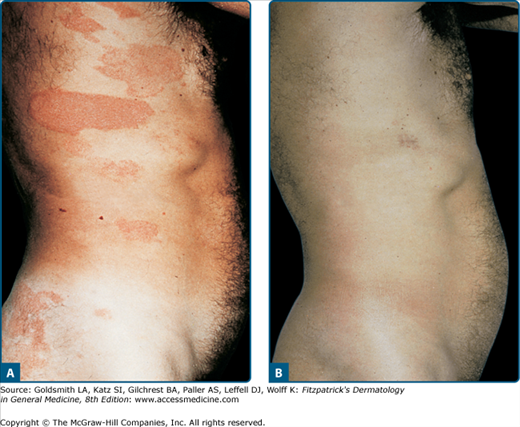
Basically all types of psoriasis respond to PUVA (Figs. 238-2 and 238-3), although the management of erythrodermic or generalized pustular psoriasis is more difficult.8 The effectiveness of oral PUVA in inducing and maintaining remission of psoriasis has been widely documented and confirmed by large-scale clinical trials (see Figs. 238-2 and 238-3).
Three studies have compared bathwater delivery of 8-MOP with oral administration.8 In two reports, initial doses were determined by skin typing, and treatments were given two to three times weekly. Dose increments were instituted with every treatment in one study, whereas smaller increments were given every third treatment in the other. In the third report, patients were treated according to the standard European regimen for oral PUVA, which is still in use (treatments four times weekly with an intermission on Wednesdays until clearing). This showed the lowest incidence of treatment failures and overdose phenomena, despite the potential burn risk of back-to-back PUVA treatments. Compared with oral 8-MOP, bath-PUVA showed equal clearing rates with fewer exposures.8 The greater therapeutic efficacy could be because of a higher penetration of psoralens through the abnormal stratum corneum overlying psoriatic plaques, as compared with healthy perilesional skin where phototoxicity is monitored during the therapy. The incidences of erythema and pruritus were similar or lower compared with oral therapy. Systemic intolerance, such as nausea and vomiting, were not observed.
Oral 5-MOP–PUVA represents an alternative to oral 8-MOP–PUVA. Psoriatic lesions are cleared with a comparable number of treatments, but at the expense of significantly higher cumulative UVA doses. This difference may be due to the lower phototoxicity potential of 5-MOP and of its higher tanning activity. However, 5-MOP–PUVA therapy is not associated with nausea and vomiting and has a lower incidence of pruritus and severe phototoxic erythema.8
On complete clearing, patients are often assigned to maintenance therapy, during which the frequency of treatments is gradually reduced. The purpose of maintenance therapy is to achieve longer remission. Maintenance therapy in the original European regimen consisted of 1 month of twice-weekly treatments, with the last UVA dose used for clearing, followed by another month of once-weekly exposures. According to other recommendations,16 maintenance treatment should be considered only on rapid relapses, because patients with a stable remission may be overtreated, and long-term risks of PUVA are related to the total cumulative phototoxic doses. In one recent left-right comparison study with psoriatics, PUVA maintenance treatment over 2 months did not increase the length of remission.17 Thus, maintenance treatment should be given only in selected cases.
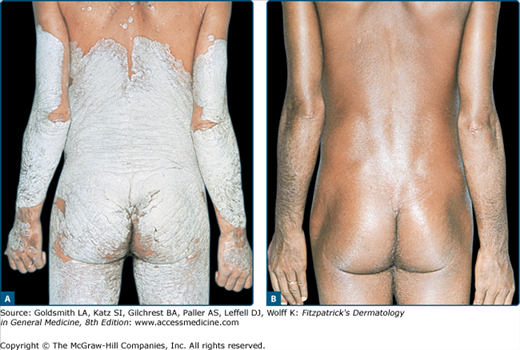
Erythrodermic and generalized pustular psoriasis (von Zumbusch type) respond to PUVA (Fig. 238-4), but the time required to induce remission is considerably longer, more treatments are needed, and higher failure rates are reported, compared with plaque or guttate varieties. Pustular eruptions of palms and soles are quite recalcitrant to treatment, regardless of whether they are true localized pustular psoriasis, nonpsoriatic palmoplantar pustulosis or pustular psoriasis, or pustular eczema. Oral PUVA alone can produce a slow but definite remission in many cases, but a considerable number of patients require adjunctive therapy for clearing. As mentioned above, the combination with topically applied 8-MOP can be beneficial, but bath-PUVA appears to be also quite effective in such cases.
PUVA alone can produce definite remissions in many patients with psoriasis, but a considerable number require additional therapies for clearing. Such combination therapy improves efficacy and may reduce side effects.
Topical adjuvant therapies with glucocorticoids, anthralin, and tar preparations, and, more recently, with calcipotriol and tazarotene, have yielded good results. However, adjunctive topical therapy is unacceptable to some patients.
A combination of PUVA and methotrexate can reduce the duration of treatment, number of exposures, and total UVA dose and is also effective in clearing patients unresponsive to PUVA or UVB alone.18 This combination appears to be safe if used during the clearing phase only. However, if used for long-term treatment, PUVA and methotrexate may act synergistically in the development of skin cancers.19
Cyclosporine plus PUVA dramatically enhances skin carcinogenesis. This is in keeping with the observation of a greatly increased risk of cutaneous squamous cell carcinomas in patients with solid organ transplants maintained on this immunosuppressant. Thus, this combination should be definitely discouraged.8,20–22
The therapeutic efficacy of PUVA therapy is dramatically increased by daily oral retinoid (etretinate, acitretin, isotretinoin; 1 mg/kg) administration beginning 5–10 days before the initiation of PUVA, and continued throughout the clearing phase. This so called RePUVA characteristically reduces the number of exposures by one-third and the total cumulative UVA dose by more than one-half. RePUVA also often clears “poor responders” who are not brought into complete remission by PUVA alone.23
The mechanism of the synergistic action of retinoids and PUVA is unknown, but may be a result of the accelerated desquamation that optimizes the optical properties of the skin and reduction of the inflammatory infiltrate. As an additional theoretic benefit, etretinate and other retinoids may protect against long-term carcinogenic effects of PUVA. In one study, patients with psoriasis treated with PUVA in combination with systemic retinoids showed a reduced risk of squamous cell carcinoma but not a significantly altered incidence of basal cell carcinoma (BCC).24 Although retinoid toxicity is generally not a concern because the administration is limited to the clearing phase, the potential teratogenicity of retinoids represents a serious concern for women of childbearing age. In these patients, the use of isotretinoin is advisable because contraception is necessary for only 1 month after discontinuation of therapy, in contrast to etretinate and acitretin, which require 2 years of contraception because of their slower elimination.8
The mechanism of action of biologic agents suggests that there may be additive effects in treating psoriasis with PUVA, but this remains to be further defined in clinical trials.25,26 Presently, there are no long-term studies evaluating the safety and efficacy of the combination of any biologics with PUVA.
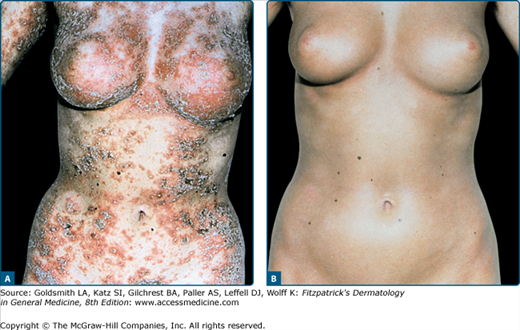
Since the first promising results with PUVA in CTCL in 1976,27 numerous investigators from the United States and Europe have confirmed the efficacy of PUVA for CTCL.28 Treatment schedules and dosimetry are essentially the same as for psoriasis. The treatment consists of a clearing phase (Fig. 238-5), a maintenance phase consisting of two exposures per week for 1 month and one exposure per week for another month, and a follow-up phase without therapy. Remission should be confirmed by histologic examination of previously involved skin sites. After therapy is discontinued, the patient is monitored monthly and later bimonthly. If a relapse occurs, the patient is again subjected to a full PUVA course. Some investigators advocate permanent maintenance treatment consisting of treatments once monthly or every other month. However, the course of CTCL varies considerably from patient to patient, and clinical experience suggests that patients benefit most from individualized treatment schedules.8
Relapses often respond as well as the initial lesions when PUVA is resumed. Clinical remissions appear to be directly related to phototoxic destruction of the malignant lymphocytes that infiltrate the skin. Thus, complete clearing may be induced when the cells are confined to the epidermis and superficial dermis, the depth of effective UVA penetration into the skin.
Present knowledge indicates that PUVA is an excellent treatment option that may induce long-lasting disease-free intervals in CTCL if used in the early stages of the disease.28 In later stages, PUVA may reduce the tumor cell burden and thus may act synergistically with other treatment. It improves quality of life and may prolong survival when used in combination with more aggressive treatment modalities. Prolonged remissions were observed with combinations of PUVA with retinoids, bexarotene,29,30 or interferon-α 2a.31–33
Patients with tumor-stage CTCL exhibit a high rate of early recurrences and, therefore, require indefinite maintenance treatment. PUVA causes complete tumor resolution only when used in combination with local x-ray treatment and/or systemic chemotherapy. Most follow-up studies have demonstrated that the great majority of patients with early disease can be kept in remission with or without maintenance therapy for several years, but tumor-stage patients (IIB) usually experience multiple recurrences despite aggressive combination therapies and eventually die within a few years.28 Currently, no therapeutic regimen is known to alter the disease course of CTCL. Psoralen UV-A is an effective treatment for MF, inducing long-term remissions and perhaps in some cases disease “cure.” Thirty percent to 50% of patients remain disease free for 10 years, but late relapses occur.34
Successful treatment of erythrodermic CTCL (Sézary syndrome) has been reported with extracorporeal PUVA (photopheresis) [see Section “Extracorporeal Photochemotherapy (Photopheresis)”]. Possible long-term hazards related to frequent PUVA treatments are better justified for patients with CTCL, compared with patients with benign conditions.
Many patients with atopic eczema can benefit from PUVA therapy.35 The treatment guidelines are the same as for psoriasis. However, the condition is more difficult to treat, and quite often a higher number of treatments are required to clear the eczema. There is a high and early recurrence rate, requiring frequent maintenance exposures. However, in a recent study, PUVA provided a better short- and long-term response than medium-dose UVA1 in patients with severe atopic eczema.36 A combination of PUVA with topical glucocorticoids appears to be superior to PUVA alone in maintaining remissions. Because the average patient is young, long-term maintenance therapy is problematic; and combination of PUVA with topical immune modulators (tacrolimus, pimecrolimus), although effective, cannot be recommended until more data are available. The mechanism of action of PUVA in atopic eczema is unclear; current concepts support an alteration of lymphocytes in the dermal infiltrate.
In generalized lichen planus, PUVA can provide an effective alternative to systemic glucocorticoid treatment, although it appears to be more resistant to PUVA than psoriasis when treated according to a similar schedule. More exposures and higher cumulative UVA doses are required for clearing, and not all patients respond satisfactorily. An exacerbation during PUVA treatment has been reported in a few patients. In patients who clear, relapses respond equally well when PUVA is resumed. Bath-PUVA can also clear lichen planus, and combined PUVA-etretinate regimen may be considered.37
In cutaneous mastocytosis (urticaria pigmentosa), PUVA leads to a temporary involution of skin lesions38 probably due to chronic degranulation of the mast cells. The treatment results in loss of Darier sign, relief of itching, and flattening and even complete disappearance of cutaneous papules and macules. Surprisingly, even systemic symptoms such as histamine-induced migraine and flushing improve gradually as treatment is continued.38 In most patients, the manifestations of the disease recur several months after discontinuation of PUVA, but the recurrences respond as well as the original lesions.
Because treatment of cutaneous mastocytosis has been unrewarding with other modalities, the use of PUVA, although not curative, seems to be warranted in patients when the disease is causing severe distress.
Both acute and chronic pityriasis lichenoides37 respond to PUVA, and favorable results have been reported for lymphomatoid papulosis.39 However, the experience with these conditions is limited to a few anecdotal cases. In pityriasis rubra pilaris, the results are quite inconsistent. Some cases seem to respond well, others may flare, and some require combination treatment with retinoid or methotrexate therapy. Generalized granuloma annulare has been reported to clear completely, but long-term maintenance treatment was required to maintain remissions.40 Regrowth of hair was noted in alopecia areata with either topical or systemic PUVA exposures localized to the alopecia areas. Follow-up studies of larger patient groups concluded that PUVA is generally not an effective treatment for alopecia areata.41 The experience of the present authors has also not been encouraging. Localized scleroderma and pansclerotic morphea have been successfully treated with bath-PUVA and oral PUVA.42,43
Acute and chronic cutaneous graft-versus-host disease (GVHD) has become indications of increasing importance. Because of the clinical and histologic similarities of idiopathic lichen planus and lichenoid GVHD, PUVA treatment was evaluated for the latter.44 PUVA cleared or improved this lichen planus-like eruption in patients who had not responded to conventional immunosuppressive therapy alone. PUVA can also improve acute GVHD,45 although results of PUVA treatment for scleroderma-like variants of cutaneous GVHD are controversial. According to our own experience, the more circumscribed, localized forms appear to respond to PUVA with softening of the fibrotic, sclerotic connective tissue, but more widespread, disseminated lesions hardly respond.
Improvement of mucosal erosions followed by healing, observed during treatment of chronic lichenoid GVHD with PUVA, suggests that PUVA may exert both local and systemic effects, but this is not proven. There is no improvement of GVHD of other organs, such as the liver. The therapeutic regimen used for the treatment of chronic GVHD is basically the same as for psoriasis. UVA doses should not be increased too aggressively, to avoid erythema and possible (re)activation of GVHD. In general, increase of the UVA dosage by 0.5 J/cm2 at maximum after every second to fourth exposure is recommended. Patients are exposed to UVA radiation three to four times weekly. After clearing of skin lesions, the frequency of exposures is reduced.
Because there appears to be an overall increased risk of secondary malignancies for all bone marrow/peripheral stem cell recipients, patients should be examined on a regular basis for the development of cutaneous malignancies, independent of whether they have been treated with PUVA.
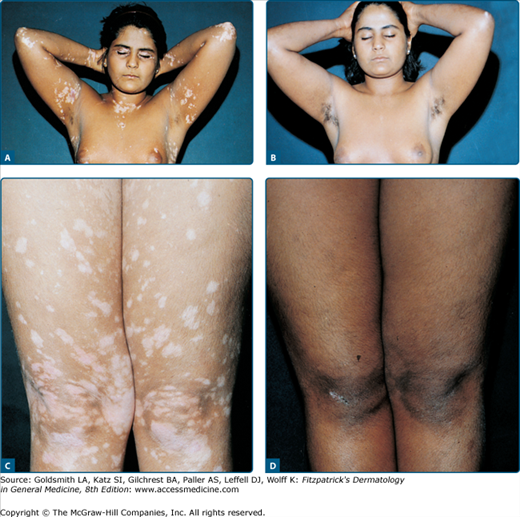
Vitiligo was the first disease treated with an ancient form of psoralen PUVA in India and Egypt. PUVA in its modern form stimulates melanogenesis, melanocyte proliferation, and migration, and can reconstitute the normal skin color in many vitiligo patients although the actual response rate has still not been defined. However, it is less used now since narrowband UVB phototherapy has been shown to be effective and possibly safer alternative for repigmentation of this condition (see later).
To induce maximal repigmentation, patients need long-term therapy with 100–200 exposures given twice or thrice weekly. Approximately 70% of patients respond after 12–24 months (Fig. 238-6), defined as the development of perifollicular macules of repigmentation. If there is no response after 6 months or approximately 50 treatments (as defined as perifollicular macules of repigmentation), PUVA should be terminated. If treatment is discontinued, this newly acquired repigmentation may be lost. The permanency of PUVA-induced repigmentation in vitiligo is poorly documented. Some investigators have reported continuing pigment loss following PUVA, while others have reported the repigmentation to be long lasting.46
Patient selection appears to be particularly important in vitiligo treatment. Lips, distal dorsal hands, tips of fingers and toes, areas of bony prominences, palms, soles, and nipples are very refractory to treatment, and patients with involvement limited to these areas should be excluded. Segmental vitiligo tends to show a variable response. Because of the different response in different body areas, total repigmentation is only rarely achieved, and some 30% of patients do not respond at all despite many months of therapy. Duration of the disease before PUVA therapy does not affect response rate.46 It should be mentioned here that in a recent trial of nonsegmental vitiligo, narrowband-UVB therapy resulted in a better color match than oral PUVA.47
The mechanisms by which PUVA induces repigmentation in vitiligo skin are speculative. However, PUVA’s known effect on a number of immunologic reactions suggests a suppression of the autoimmune stimulus for melanocyte destruction.
Tolerance to sunlight can be induced in several photodermatoses by PUVA therapy.48 In polymorphous light eruption, the most common photodermatosis, PUVA is the most effective preventive treatment.49 In approximately 70% of patients with this condition, a 3- to 4-week PUVA course of two to three treatments per week suffices to suppress the disease on subsequent exposure to sunlight such as a holiday trip or the arrival of summer. The initial exposure and dose increments during therapy should be performed according to the guidelines outlined for psoriasis. PUVA has the advantage of a rapid and intense pigment induction at relatively low UVA doses that usually remain well below the threshold doses for eliciting the rash. Approximately 10% of patients develop typical lesions during the initial phase of PUVA, but these usually disappear when treatment is continued. The authors’ treatment schedule consists of three to four treatments per week for 3–4 weeks in early spring. PUVA protects only temporarily, but subsequent sun exposures usually maintain protection and many patients remain protected for 2–3 months even after their pigmentation has faded.
The mechanism by which phototherapy induces tolerance to sunlight is not clear. Hyperpigmentation and thickening of the stratum corneum may be important factors, but other mechanisms, such as modulations of cutaneous immune function, may also be involved.49
There is also some experience with PUVA prophylaxis of other photodermatoses. In solar urticaria, PUVA therapy appears to be the most effective preventive treatment available and is certainly better than antihistamines. Tolerance to sunlight can be increased 10-fold or more after a single treatment course.50 The suppressive effect may last throughout the summer if the patients have regular sunlight exposures, which seems to be necessary to maintain tolerance. Problems may arise during the first PUVA exposures, because, in some patients, the urticaria threshold dose is very low. In these cases, careful conditioning by stepwise UVA irradiation of single quadrants of the body surface a few hours before each PUVA treatment has proved useful. Treatments with PUVA are then given during the refractory period of presumed mast cell degranulation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree