Pharmacokinetics and Topical Applications of Drugs: Introduction
|
Pharmacokinetics related to topical applications of drugs describes the time-dependent drug concentration following the application of the drug to the skin surface, its subsequent passage through the skin barrier into the underlying skin layers, and its distribution into the systemic circulation. The subject continues to hold the attention of research scientists and clinicians alike because of its relevance to dermatologic therapy and the possibility of topical application of current systemic medication that cannot be administered orally, such as peptides or proteins. However, this is an inherently complex subject, despite the advent of new insights into the principal factors that govern diffusion of a drug into and across the skin.
The major difficulty in developing an accurate description of the percutaneous absorption of a drug is related to the size of the compartments. A topical application of a cream or ointment is generally spread to a thickness of not greater than 10 μm. The thickness of the stratum corneum is also approximately10 μm, whereas the viable epidermis, dermis, and, to a greater extent, the systemic compartment, represent a large sink in which absorbed drugs undergo dilution to levels that often remain undetectable to all but the most sensitive techniques. The determination of the time-dependent changes in the concentration of a drug in individual compartments is technically challenging. After topical application of a drug formulation, several parameters can affect this process (Box 215-1).
|
Diffusion
Compounds applied topically to the skin surface migrate along concentration gradients according to well-described laws governing diffusion of solutes in solutions and/or their diffusion across membranes. For a detailed discussion of relevant equations, readers are referred to several comprehensive reviews.1–4
Diffusion of uncharged compounds across a membrane or any homogeneous barrier is described by Fick’s first and second laws. The first law J = −D (ΔC/Δδ) states that the steady-state flux of a compound (J = moles/cm/s) per unit path length (δ, cm) is proportional to the concentration gradient (ΔC) and the diffusion coefficient (D, cm2/s). The negative sign indicates that the net flux is in the direction of the lower concentration. This equation applies to diffusion-mediated processes in isotropic solutions under steady-state conditions. Fick’s second law predicts the flux of compounds under nonsteady-state conditions. Diffusion is an effective transport mechanism over very short distances, but not over long ones. The relationship between the time (Δt) it takes for a molecule to migrate along a path length (x) and its diffusion coefficient is governed by the equation:
Δt = x2/2D.
For example, the diffusion coefficient for water in an aqueous solution is: 2.5 × 10−5 cm2/s, suggesting that a water molecule would migrate over a 10-μm path (the equivalent of the width of the stratum corneum) in 0.4 ms. However, because diffusion depends upon the square of the distance, longer distances are not efficiently covered; a 100-μm path would take 40 ms.
Three-Compartment Model
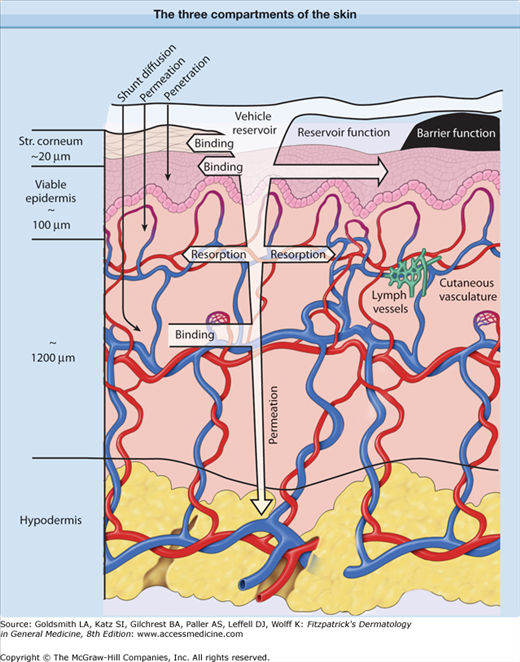
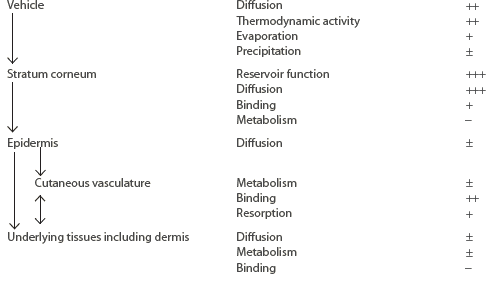
Although pharmacokinetic analysis of topical preparations may require the description of a relatively large number of compartments, this discussion is confined to the three outlined in Figure 215-1: (1) the skin surface, (2) the stratum corneum, and (3) the viable tissue. The formulation itself forms a reservoir, from which the compound must be released; in order to undergo percutaneous absorption, the compound then must penetrate the stratum corneum, diffuse into and through the viable epidermis into the dermis, and, finally, gain access to the systemic compartment through the vascular system. In addition, the substance may diffuse through the dermal and hypodermal layers to reach underlying tissues. As summarized in Table 215-1, within each compartment, the compound may diffuse down along its concentration gradient, bind to specific components, or be metabolized. The size or characteristics of each compartment may alter with time, and the factors determining diffusion within each compartment may be affected by disease state as well as the nature or the pharmacologic/biologic activity of the drug or its excipients.
Figure 215-1
Diagrammatic representation of three compartments of the skin: surface, stratum (Str.) corneum, and viable tissues. After surface applications, evaporation and structural/compositional alterations in the applied formulation may play an important role in determining the bioavailability of drugs. The stratum corneum, the outermost layer, plays the most significant role in determining the diffusion of compounds into the body. After absorption, compounds may bind or diffuse within the viable tissues, or become resorbed by the cutaneous vasculature.
Compartment | Processes | General Relevance to Bioavailability |
|---|---|---|
| ||
Formulations differ in their physicochemical properties, and, as discussed in Section “Formulations,” this influences the kinetics of release and/or absorption. However, the principal consideration is that topically applied drug products represent a physically small compartment, which limits the amount of compound that can be applied to the skin surface. When a patient applies a dermatologic preparation, the layer of a formulation covering the skin is very thin (approximately 0.5–2.0 mg/cm2). Thicker layers are felt as “unpleasant” and are consciously or subconsciously rubbed off or spread to larger surfaces. This restricts the amount of an active compound that can effectively come in contact with the skin surface to approximately 5–20 μg/cm2 for a 1% (wt/wt) topical formulation.
However, even after being rubbed in, formulations do not remain homogeneous over the time course of penetration. For example, topical applications containing water, alcohol, or similar solvents undergo rapid evaporation.5 This phenomenon is readily recognized by patients as a cooling sensation. The evaporation results in rapidly increasing concentrations of nonvolatile substances on the skin surface, which may result in the formation of supersaturated “solutions” or, alternatively, precipitation of active ingredients. Formulations may also mix with skin-surface lipids or undergo time-dependent changes in their composition as excipients and drugs undergo absorption. Altogether, these considerations suggest that dramatic changes in the composition and structure of formulations may occur following surface application, all of which may determine the subsequent bioavailability of active ingredients.
The reservoir function was first described by Vickers,6 who observed that simple occlusion leads to the renewed onset of a glucocorticoid-mediated vasoconstriction several hours after it had declined. He interpreted this effect as renewed liberation of the glucocorticoid from a “reservoir” stored in the upper skin layers.
We define as reservoir the amount of an active ingredient that is still in contact with the nonvolatile constituents of its formulation after the latter had been massaged into the skin surface. The compound has not yet penetrated, but it cannot be removed by simple rubbing or contact with clothing or other tissues. The reservoir thus adheres to the skin surface and resides in the wrinkles and the upper layers of the stratum corneum. Reservoirs on eczematous skin may become even more prominent because of the scaliness of the skin. Recently, we discovered that the upper volume of the follicular channels serves also as a reservoir, which may result in a relative increase in absorption through appendages. In-vivo laser scanning microscopy measurements found that the hair follicles represent an efficient reservoir for topically applied formulations, which can be compared with the reservoir of the stratum corneum on several body sites.7,8 This phenomenon may be increased in formulations that contain particles or precipitates, given the evidence that appropriately sized particles can rapidly penetrate along the shafts of hair follicles to a depth of up to 100–500 μm.9–12
The optimum size of the particles for penetration into hair follicles is between 300 and 600 nm, which corresponds to the cuticular structure of the hairs.13,14 It was assumed that the rigid hair shaft acts as a geared pump, because this effect could only be observed in the case of moving hairs.14 The follicular reservoir may result in a relative increase in the absorption of topically applied substances. No evidence has been found that topically applied substances penetrate efficiently into the sweat glands. This may be due to sweat outflow or other, unknown reasons.
Formulations can be differentiated on the basis of whether they are designed to remain on the skin surface (sunscreen products and cosmetics), to be delivered to compartments in the skin (topical formulations), or to migrate across the skin into the central compartment (transdermal formulations).
Formulations may affect the kinetics and the degree of percutaneous absorption and, subsequently, the onset, duration, and extent of a biologic response. In the context of percutaneous absorption, there are several different parameters that should be considered when selecting a formulation1,15: the thermodynamic activity of the active ingredient16; the amount of compound that can be incorporated into the formulation17; the stability of the formulation on the skin surface (e.g., emulsions may break easily)18; the partition coefficient of the active ingredient between the vehicle and the stratum corneum19; and the enhancer activity.
In general, percutaneous absorption is proportional to the thermodynamic activity of the compound. Thus, the greatest flux is observed at the active ingredient’s maximum solubility in a vehicle. Vehicles that are very good solvents should be avoided because they may retain the active ingredient on the skin surface.
Liposomes are microscopic spheres comprising a bilayer that encloses an inner aqueous core. A wide variety of cosmetics contain liposomes. Liposome-based formulations have proved to be safe, cosmetically attractive, and well accepted. There is considerable evidence that, at least for some preparations, application of liposomes is mildly occlusive and improves the hydration level of the stratum corneum. Interest in the use of liposomes to enhance the delivery of drugs across the skin has been spurred by several observations in animal models: liposome formulations were believed to enhance the penetration of compounds across the skin or to optimize the retention of bioactive compounds in target tissues.20 However, these early studies, which relied largely on animal models, were followed by relatively few in-vivo studies for humans17 conducted under standard conditions.
The action mechanism of liposomes is based on a partly damaged liquid layer of the stratum corneum, so that the liposomes can penetrate efficiently into the skin barrier. Deep in the stratum corneum, the liposomes get damaged and release their drug, which has to pass through the last cell layers of the stratum corneum by itself to reach the living cells.21
There is no clear evidence that liposomes can pass the skin barrier as intact structures, but intact liposomes can penetrate along the hair shaft and this route may be appropriate for delivery of bioactive compounds into sebaceous glands or hair follicles.7,8 Rigid liposomes penetrate better into the hair follicles than flexible liposomes, which supports the assumption that the moving hairs act as a geared pump.21
(See Chapter 47.)
The primary compartment that limits the percutaneous absorption of compounds is the stratum corneum. This thin (10–20 μm) layer effectively surrounds the body and represents a highly differentiated structure that determines the diffusion of compounds across the skin. The physical description of the stratum corneum is well documented,22 and it can be accurately described as “bricks” of bundled, water-insoluble proteins, embedded in a “mortar” of intercellular lipid.
The stratum corneum is a highly organized, differentiated structure. To participate fully in forming an effective barrier to diffusion, the biogenesis of the corneocytes as well as the synthesis and processing of the intercellular lipid must proceed in an orderly manner. Disruption in the kinetics of skin barrier formation by accelerating the division of the keratinocytes in the underlying layers will lead to a disruption in the barrier properties of the skin.23 Thus, the concept of dead or dying skin forming a passive barrier to diffusion is now replaced by a model of the stratum corneum as a highly differentiated structure with unique properties that are particularly suited for its role in forming the skin barrier (see Chapter 47).24
A variety of appendages penetrate the stratum corneum and epidermis, facilitating thermal control and providing a protective covering. Appendages are potential sites of discontinuity in the integrity of the skin barrier. The density of the hair follicles varies on different body sites. Hair follicles represent a reservoir that may store topically applied substances. A detailed analysis of the reservoir of the hair follicles showed that the highest reservoir is on the scalp, followed by the forehead and the calf.25 On the forehead, there are a high number of small follicles, while the calf contains fewer but larger hair follicles. These reservoirs are comparable to the reservoir of the stratum corneum on these body sites. The percentage of the hair follicles on the total skin surface varies between 0.2% and 1.3%, depending on the body site.25 Differences in the follicular penetration were observed in different ethnic groups.26 Hair follicles appear to present an important pathway for percutaneous absorption in nondiseased skin.11,12 This can be explained by the fact that only the upper wall of the follicular apparatus (the acroinfundibulum) is protected by a coherent stratum corneum, whereas in the lower part (infrainfundibulum), the corneocytes appear undifferentiated, and protection is incomplete, if not absent. Even solid particles may enter deep into the follicular orifice,9,10,22 a phenomenon that lends itself to the concept of follicular targeting of drugs.22
It follows that in relationship to the integral protection against the passage of xenobiotics in general, and drugs specifically, the barrier function of the interfollicular stratum corneum is even more potent than previously believed, whereas more research is needed relative to the follicular pathway. Recent investigations hint to the presence of active follicles (open to penetration) and passive ones.11
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree