Pediatric Kidney Transplantation
Peter L. Abt
DEFINITION
Kidney transplantation is the preferred method of renal replacement therapy in children. Compared to dialysis, advantages of kidney transplant include prolonged survival, restitution of physical and cognitive development, and lifestyle restoration. Kidney transplantation in children is challenged by organ availability, compliance with postoperative immunosuppression, and disease recurrence.
PATIENT HISTORY AND PHYSICAL FINDINGS
Pediatric kidney transplants can only be performed at accredited transplant centers.
Candidates for kidney transplantation are evaluated by a multidisciplinary team consisting of a surgeon, pediatric nephrologist, nutritionist, social worker, financial counselor, and psychiatrist. Depending on the etiology of the underlying renal disease and associated comorbidities, an interdisciplinary approach to evaluation often includes urology, cardiology, and hepatology.
Transplantation is indicated for those individuals who are already on dialysis or approaching the need for dialysis. For those patients who are not yet on dialysis, an absolute glomerular filtration rate, which defines a threshold to initiate transplant does not exist. Rather, signs and symptoms of uremia, growth retardation, and cognitive changes should guide the timing of transplant.
A thorough history should be performed. In particular, any history of genitourinary surgery, dialysis access, hypercoagulability, cardiovascular, or liver disease should be explored. A family history of kidney disease should be obtained as this may impact siblings or indicate associated abnormalities in the recipient.
Recent treatment for malignancy and ongoing substance abuse are contraindications to transplant. A list of absolute and relative contraindications is shown in Table 1.
Table 1: Absolute and Relative Contraindications to Renal Transplant
Absolute Contraindications
Relative Contraindications
Active malignancy or a period of <12 mo after treatment
Active infection
Irreversible debilitating brain injury
Active substance abuse
Severe multisystem failure
ABO incompatibility with the donor
Positive T- and/or B-cell crossmatch
HIV infection
Long-standing medical noncompliance
Severe psychomotor delay
Psychiatric illness
Inadequate social support
Active autoimmune diseases
A full physical should be performed, with particular attention for signs of growth retardation, prior dialysis access in the femoral or iliac vasculature, vascular insufficiency to the lower extremities, and prior abdominal surgery. Evidence of diminished pulses in the lower extremities or lower extremity edema are indications of potential inflow and outflow abnormalities requiring further study.
Previous genitourinary evaluation and surgical operative reports should be reviewed. Reconstructive surgery may alter the anatomy, impact bladder urodynamics, and require alternatives to standard bladder drainage in the recipient.
Potential living kidney donors should be encouraged to undergo evaluation. These individuals are evaluated separately from the recipient.
IMAGING AND OTHER DIAGNOSTIC STUDIES
The donor must be ABO-identical or compatible with the recipient, unless in an ABO-incompatible protocol. Tissue human leukocyte antigen (HLA)-typing and crossmatching should be performed. If the preliminary crossmatch is positive with a living donor, kidney exchange programs and desensitization protocols may be an option.
Recipients should be evaluated for anemia and metabolic perturbations such as hyperkalemia and acidosis. These should be corrected prior to proceeding with transplant.
Laboratory examination includes electrolytes; complete blood count; liver function tests; and serologies for hepatitis B and C, HIV, cytomegalovirus (CMV), and Epstein-Barr virus (EBV). BK virus testing is necessary in retransplant candidates. Urine should be tested for protein content and cultures performed. All candidates should receive a chest x-ray.
Imaging of the aorta and iliac arteries and their venous counterparts is necessary if there is suspicion of congenital vascular anomalies or evidence of prior interventions. A history of spina bifida, complex surgical repair for cloacal exstrophy, or dialysis access in the lower extremities or iliac or caval system should decrease the threshold for imaging to ensure patency of the venous system. Duplex studies are usually adequate, but computed tomography (CT) scans or magnetic resonance imaging (MRI) may be necessary.
SURGICAL MANAGEMENT
Preoperative Planning
The operative approach is planned based on the size of the child. A retroperitoneal approach is appropriate for most children greater than 30 kg. For those less than 20 kg, the kidney is usually too large for retroperitoneal placement and should be located within the peritoneal cavity. Between 20 and 30 kg, the size of the kidney and the child dictates variation in the operative approach.
Intraoperative initiation of immunosuppression usually includes induction therapy; however, this will vary with the immunologic status of the child, degree of HLA matching with the recipient, and transplant program-specific protocols.
Consent of the recipient should include operative risks such as graft thrombosis, urinary leak, bleeding, rejection, and complications from immunosuppression.
Positioning
Kidney transplantation is performed in the supine position. A Foley catheter with a dual lumen or one with a single lumen with a “Y” connector should be placed. This allows retrograde filling of the bladder to facilitate the ureteroneocystostomy.
A central venous catheter and arterial line are necessary in smaller children to guide volume administration and monitor acid base status. In some circumstances, a central line may be required for the administration of induction immunosuppression.
Sequential compression devices should be used in larger children.
Prior to making an incision, ABO compatibility between donor and recipient and United Network of Organ Sharing donor identification number should be confirmed.
TECHNIQUES
BACKBENCH OF THE KIDNEY
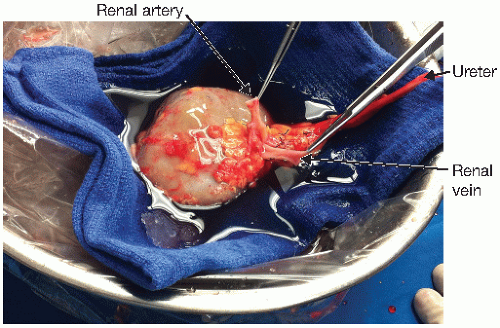
The kidney is prepared under sterile conditions in preservation solution, which is chilled on ice (FIG 1).
The perinephric fat is removed and the parenchyma inspected. Discoloration and complex cysts should be noted and may require further investigation. Biopsy sites can be closed with 3-0 silk or 4-0 Prolene.
The artery and veins are inspected for accessory vessels, anatomic abnormalities, and trauma, which may have occurred prior to or at the time of donation. Both the artery and vein are cleared of surrounding tissue with sharp dissection. Retroperitoneal, adrenal, and gonadal branches are ligated and divided. Carrel patches of aorta and vena cava can be created.
The ureter is inspected for anatomic abnormalities. As with the vasculature, it is not uncommon to observe more than one ureter. Limited dissection of the ureter should be conducted in order to preserve its blood supply, in particular, the triangular area between the lower pole, ureter, and renal artery.
Arterial and venous reconstruction may be necessary in cases of multiple vessels.
RETROPERITONEAL APPROACH—EXPOSURE OF THE ILIAC VASCULATURE
This approach is appropriate for children greater than 30 kg and can be used for those children between 20 and 30 kg if the child’s morphology and size of the kidney allow adequate space for locating the kidney. Placement of a kidney on the left iliac system follows a similar approach to that of the right-sided approach described here.
A right lower quadrant incision is made using the pubic symphysis and right anterior superior iliac crest as landmarks. The layers of the abdominal wall are divided with electrocautery to enter the preperitoneal space. The round ligament in females and the inferior epigastric vessels in all patients may be ligated and divided between 2-0 silk ties to facilitate exposure of the retroperitoneum.
The peritoneum is peeled off the lateral pelvic side wall; avoid rents in the peritoneal membrane. This will allow exposure to the vasculature. A Bookwalter retractor provides exposure (FIG 2A).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree