31 Pediatric facial fractures
Synopsis
 Traumas that would likely produce fractures in adults often do not in children due to intrinsic anatomical factors.
Traumas that would likely produce fractures in adults often do not in children due to intrinsic anatomical factors.
 In addition to the unique anatomy of the pediatric patient, future growth and development must be accounted for when addressing these injuries.
In addition to the unique anatomy of the pediatric patient, future growth and development must be accounted for when addressing these injuries.
 A growing appreciation of the pediatric craniofacial skeleton’s resilience and the utility of late orthodontics is making practitioners more comfortable with conservative strategies.
A growing appreciation of the pediatric craniofacial skeleton’s resilience and the utility of late orthodontics is making practitioners more comfortable with conservative strategies.
 In deciding between operative and conservative management of pediatric facial fractures, the practitioner is essentially weighing the risk of growth disturbance against the benefit of precise reduction and rigid fixation.
In deciding between operative and conservative management of pediatric facial fractures, the practitioner is essentially weighing the risk of growth disturbance against the benefit of precise reduction and rigid fixation.
 Craniofacial development, along with the resilience of the pediatric skull and supporting structures, often facilitates a less invasive approach to managing these complex injuries.
Craniofacial development, along with the resilience of the pediatric skull and supporting structures, often facilitates a less invasive approach to managing these complex injuries.
Introduction
Facial fractures are relatively uncommon in children. Traumas that would likely produce fractures in adults often do not in children due to intrinsic anatomical factors such as larger fat pads, decreased pneumatization of sinuses, increased skeletal flexibility secondary to more malleable bone stock, and compliant sutures. Parental supervision also prevents many would-be fractures.1 The same structural characteristics of the pediatric craniofacial skeleton that thwart fracture are responsible for the unique injury patterns that are observed when bony injury does occur. Pediatric facial fracture evaluation begins with the ABCs of trauma and proceeds through clinical and radiographic assessment, culminating in conservative or operative management. In addition to the unique anatomy of the pediatric patient, future growth and development must be accounted for when addressing these injuries. A conservative approach is advised whenever feasible, and long-term follow-up is mandatory to ensure adequate outcomes and inform future practice. The objective of this chapter is to provide the reader with an understanding of the anatomical and growth-related factors that make pediatric craniofacial fracture repair a unique entity, and to offer a discussion of specific treatment modalities in this context.
Historical perspective
Advances in facial fracture prevention include progress in automotive technology, personal protective equipment in sports, and the introduction of speed limits, alcohol, cellular phone and text-messaging legislation. Improved imaging modalities have facilitated the recognition of distinct craniofacial injury patterns in children: a relatively contiguous facial skeleton, uninterrupted by large sinuses, is clearly appreciated on two-dimensional (2D) and 3D computed tomography (CT).2,3 Management strategies are incorporating biomaterials increasingly compatible with craniofacial growth and development. A growing appreciation of the pediatric craniofacial skeleton’s resilience and the utility of late orthodontics is making practitioners more comfortable with conservative strategies. A better understanding of the implications of fracture management on craniofacial growth and development is developing as more long-term follow-up data are generated.
Basic science/disease process
Incidence
Pediatric facial fractures comprise less than 15% of all facial fractures and increase in frequency with age.4,5 One review found 54% of fractures to occur in the skull, one-third in the upper and middle thirds of the face, and the remainder in the lower third.6 In the authors’ cohort, orbital fractures were the most common across all age groups.7 Pediatric orbital fractures represent anywhere from 3% to 45% of facial fractures.8–11 Midface fractures are uncommon (10.4% of facial fractures in the authors’ series), likely secondary to the protection of the midface by the prominent forehead and mandible in children, in addition to the robust anatomy of this region.12 Zygomaticomaxillary complex (ZMC) fractures are the most common of the rare midface fractures.1 Nasal fractures comprise up to 50% of pediatric facial fractures.8,13 Nasal and maxillary fractures were the most common osseous injury among infants in the US National Trauma Databank, while mandible fractures supplanted these in older teenagers, with mandible fractures being the overall most common facial fracture.5 Mandible fractures are often cited as the most common pediatric facial fracture, accounting for 20–50% of all pediatric facial fractures.8,11,14,15 The prominence of this structure explains these findings. Of mandible fractures in the authors’ series (n = 179), condylar head and subcondylar fractures were most common (48%).16 Anatomical distribution varies with age; condylar fracture incidence decreases, while body and angle fractures increase.17
Demographics
In the authors’ series,7 there was a 69%:31% male-to-female ratio. A total of 62.6% of patients were admitted to hospital, and 18.6% to an intensive care unit. In all, 35.9% of facial fractures underwent operative management. Half (48%) of fractures were sustained by patients 12–18 years of age; 6–11-year-olds accounted for 32% of fractures and children under 5 years of age contributed 20% of fractures.7 Similar patterns have been observed by other authors.1,4,5,18 Per the US National Trauma Databank, motor vehicle collision (MVC), assault, and falls were the most common cause of facial fracture across all age groups.5 In the context of MVC, unrestrained children were significantly more likely to sustain facial fractures: 65–70% of children involved in all-terrain vehicle or bicycle accidents were not wearing helmets.5 In the authors’ series, the cause of injury varied by age: violence, assault, and MVC were the most common causes of injury in 12–18-year-olds, while activities of daily living caused the most fractures in 0–5-year-olds. Orbital fractures were the most common fracture type across all age groups. Naso-orbital ethmoid (NOE) fractures were the least common.7
Associated injuries
Facial fractures are high-energy injuries, and as such are heavily associated with other injuries. The authors conducted a unique review of all patients with International Statistical Classification of Diseases 9 (ICD-9) codes indicative of facial fracture presenting to the emergency department of their center (n = 782). Children were included regardless of whether they were treated operatively or conservatively, or as inpatients, outpatients, by plastic surgery, or by any of the other services handling facial traumas.7 The goal was to improve patient capture and remove selection bias that may otherwise have been present due to admission status, treating specialty, or need for operative intervention. In this series, excluding soft-tissue injuries, brain trauma was the injury most commonly associated with facial fractures across all age groups. A total of 55% of patients with facial fractures had associated injuries: 81% of these were considered “serious” and included cardiovascular, cervical spine, or intra-abdominal trauma. Some 47% had neurological injuries (60% of these were concussions), and 3% had ophthalmologic injuries, including blindness. In all, 1.4% died as a result of their injuries.
Growth and development
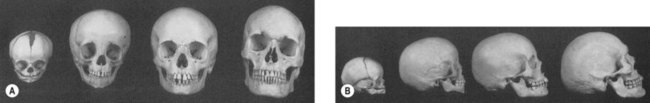
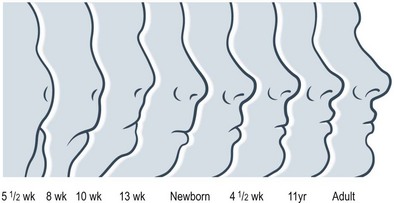
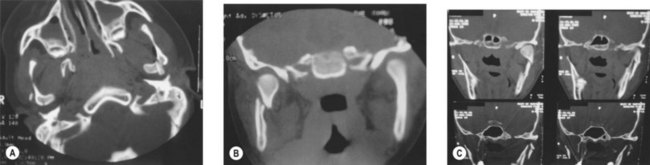
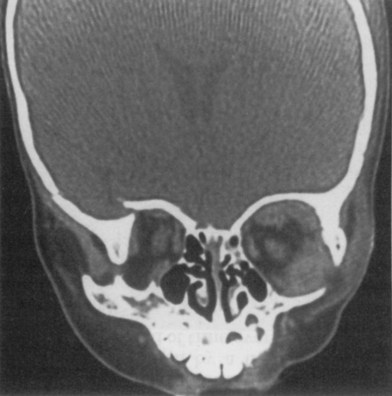
Craniofacial development is the culmination of a complex and incompletely understood interaction between intracellular processes, intercellular signaling, and environmental influences. Cranial-to-facial ratio decreases with maturity from 8 : 1 at birth to 2 : 1 in adulthood19 (Figs 31.1 and 31.2). The cranium grows secondary to the brain, which triples in size during the first year of life.19–22 This is a continuous process that is 25% complete at birth, 75% complete at 2 years, and 95% complete by 10 years. Facial growth is sporadic: it is 40% complete at 3 months, 70% at 4 years, 80% by 5 years, pauses until puberty, and resumes until 17 years of age.19 The upper face grows secondary to brain and ocular development; midfacial growth follows the development of the nasal capsule and dentition. Orbital growth is complete by 6–8 years and nasal growth is largely complete by 12–14 years. The palate and maxilla achieve two-thirds adult size by 6 years.23 At birth, the mandible is formed by two bones joined by cartilage at the symphysis, ossifying within the first year of life. The majority of permanent teeth erupt by 12 years of age. The gonial angle becomes increasingly acute, the ramus and body enlarge, and the distance between the dentition and the inferior mandibular border increases. Cortical bone replaces tooth buds as the primary component of mandibular volume. The inferior alveolar nerve is displaced superiorly to rest midway from the superior and inferior mandibular borders. The mental foramen migrates to rest ultimately beneath the first or second permanent premolar.24
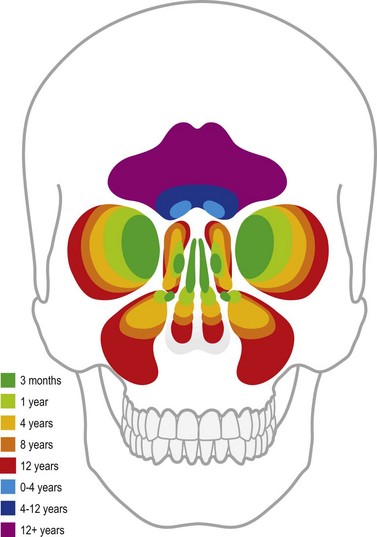
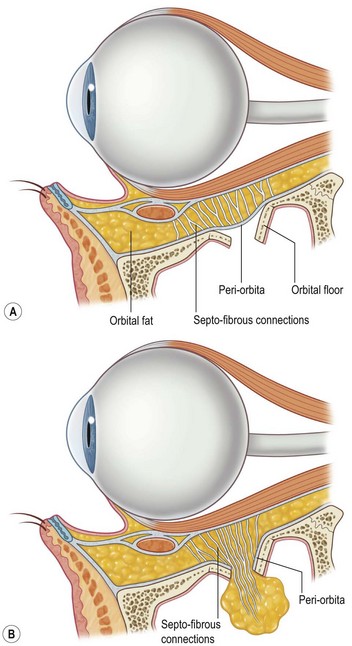
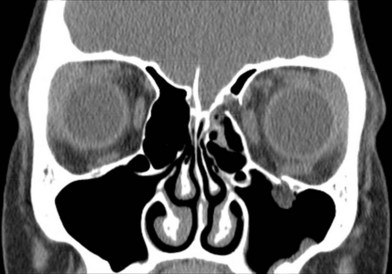
The decreased bone mineral content of the infant skull yields increased tolerance to force without fracture; fractures that do occur are more likely to be incomplete “greensticks.” In addition to mineralization, sinus pneumatization and dental eruption are responsible for the evolving craniofacial load-bearing capacity and subsequent fracture patterns (Figs 31.3 and 31.4). The maxillary sinus is aerated at 12 years of age; the frontal sinus is not aerated until adulthood. Oblique craniofacial fractures precede the Le Fort patterns seen in adulthood as an incompletely pneumatized frontal sinus transmits energy directly from the site of impact to the supraorbital foramen and then to the orbit and zygoma. In one large study, Le Fort fractures were only seen in patients greater than 10 years of age.18 Forehead fractures in children may develop into growing skull fractures (documented in 0.6–2% of pediatric skull fractures).25 These lesions develop secondary to brain pulsations transmitting through occult dural disruptions and driving a growing bony diastasis. Another consequence of the underdeveloped frontal sinus is an increased incidence of isolated orbital roof “blow-in” fractures.26 Blindness also occurs with increased frequency due to the direct transmission of force to the orbit.26 Trapdoor fractures are more common secondary to greater bony elasticity.27 Children tend to have fractures without enophthalmos or vertical orbital dystopia (VOD), likely secondary to more robust supporting structures existing in this population. Enophthalmos and VOD require composite injury to bone, ligaments, and periosteum allowing for an increase in intraorbital volume (Fig. 31.5).
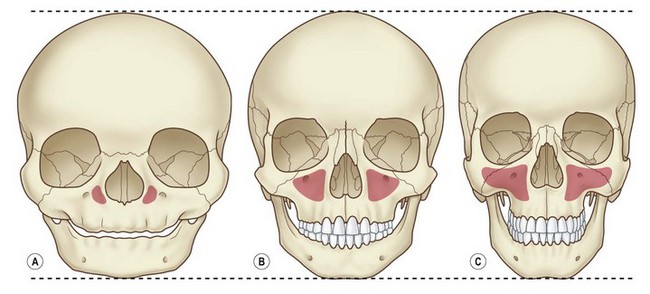
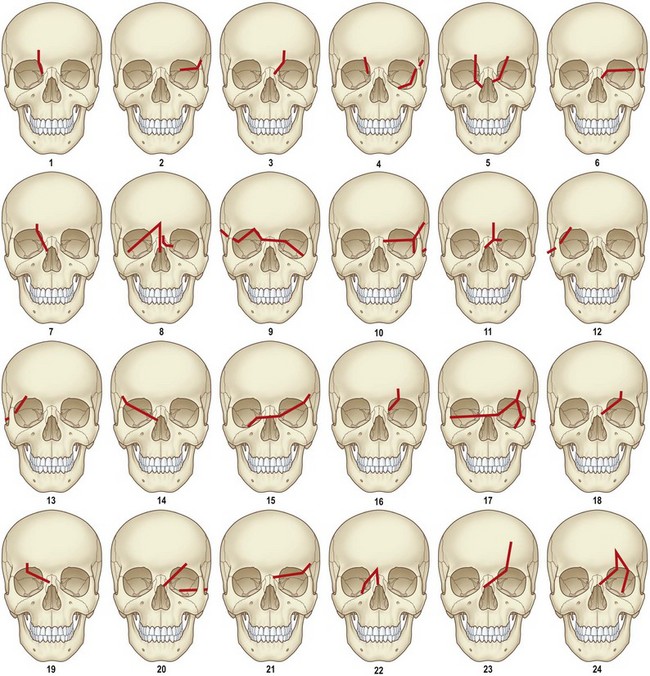
Isolated midface fractures are rare in children as this region is shielded by the prominent forehead and mandible.28 Palatal splits are more common secondary to incomplete ossification of the hard palate. These injuries represent significant potential for growth disturbances secondary to the presence of growth centers in the maxilla and nasal capsule and because the midface is retruded relative to the cranium at this age.8,29,30 Incomplete zygomaticofrontal (ZF) suture union leads to fracture dislocations characterized by inferior displacement of the zygoma and orbital floor, further contributing to oblique fracture patterns.23 Oblique fracture patterns are also encouraged by the underdevelopment of the midface skeleton and major buttress systems. Until 10 years of age, the underdeveloped maxillary sinus transmits force to the alveolus, resulting in alveolar fractures instead of Le Fort I fractures. Le Fort IIs are replaced by unilateral NOE fractures. Le Fort IIIs are replaced by multifragment oblique craniofacial fractures.18 The authors have consistently seen oblique fracture patterns in their patients (Figs 31.6–31.8).31
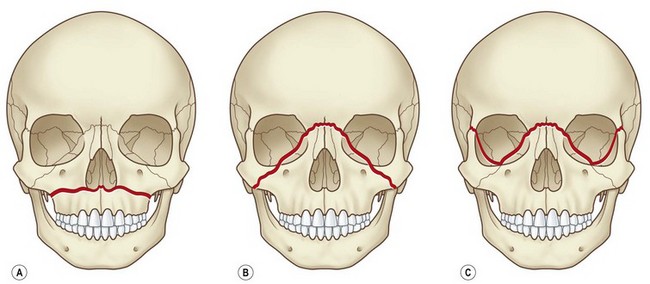
Fig. 31.6 These are the classic Le Fort fracture patterns described in adult craniofacial fractures: (A) Le Fort I; (B) Le Fort II; (C) Le Fort III. Children exhibit distinct fracture patterns, as described in the text and demonstrated in Figures 31.7 and 31.8.
The less mineralized, more compliant pediatric mandible is more resistant to comminuted fractures. Condylar head and subcondylar fractures are seen more frequently in children due to incomplete ossification and a relatively weak condylar neck (Figs 31.9 and 31.10). While certain regions of the mandible are classically highlighted as growth centers (e.g., condyles and lingual tuberosity), condylectomy and differential masticatory strain studies point to a more diffuse, dynamic process by which morphological change proceeds via coordinated bone deposition and resorption.24 Heightened awareness of potential growth disturbance and temporomandibular joint (TMJ) ankylosis is, however, justified in the setting of condylar fractures given an incomplete understanding of these injuries’ implications.
Diagnosis and presentation
The importance of a consistent approach to craniofacial trauma cannot be overemphasized. The first step in treating acute craniofacial injuries is ensuring that the trauma ABCs have been completely addressed. The craniofacial surgeon’s most direct interaction with this process will likely be in assuring a secure airway in cases where anatomy has been severely distorted. While infants are obligate nasal breathers, their nasal airway is relatively narrow and thus easily obstructed.32 Meticulous hemostasis must be achieved given a child’s relatively decreased blood volume and ability to mask significant losses with normotension prior to rapid decompensation.32,33 Hypothermia is also more likely to be problematic given a child’s increased surface area-to-volume ratio.32
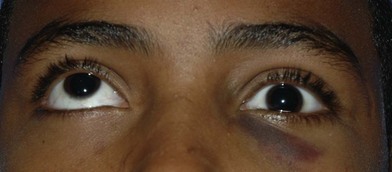
A systematic physical exam is performed. Eyelid hematoma, hearing loss, hemotympanum, and cranial nerve (CN) palsy may herald skull base fractures. Exophthalmos and inferior globe displacement may represent a supraorbital or roof fracture. Ptosis may be present secondary to levator paralysis. Orbital trauma will likely be accompanied by periorbital echymosis and subconjunctival hematoma. Extraocular muscle restriction may cause diplopia (Figs 31.11 and 31.12). Forced duction is required to rule out muscle entrapment in obtunded patients. Superior orbital fissure syndrome (internal and external ophthalmoplegia (CN II, IV, VI paralysis), proptosis, and CN V paresthesia) and orbital apex syndrome (superior orbital fissure syndrome with blindness secondary to CN II involvement) must be emergently addressed (Fig. 31.13). In nasal-orbital-ethmoid fractures, a bowstring test (palpation of the bony medial canthal attachment on lateral distraction of the lower eyelid) will assess the integrity of the medial canthal tendon. Intraorbital distance is assessed to exclude traumatic telecanthus. Gaze limitations – even if other clinical signs and symptoms are minimal and radiographic studies are equivocal – may represent entrapment in an entity termed the “white-eyed blowout fracture.”34
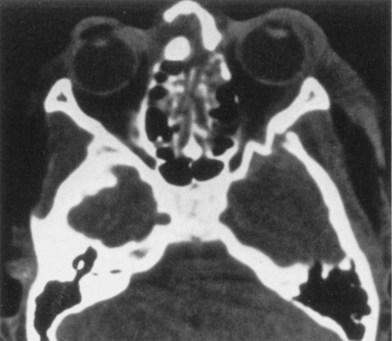
Maxillary mobility and malocclusion may represent midface fractures. ZMC fractures may be accompanied by upper buccal sulcus hematoma, epistaxis secondary to a fractured maxillary sinus, a preauricular depression, cheek flattening, or lateral canthal dystopia. Impingement of a depressed zygomatic arch on the coronoid process may yield trismus. Medial lateral wall displacement with subsequent decrease in orbital volume may yield exophthalmos (Fig. 31.14). Nasal deviation, compressibility of the nasal dorsum, and septal hematoma must be appreciated on exam. Nasal airway obstruction may represent septal hematoma.
Patient selection
In deciding between operative and conservative management of pediatric facial fractures, the practitioner is essentially weighing the risk of growth disturbance against the benefit of precise reduction and rigid fixation. Below is a fracture-specific discussion of anatomical and developmental factors to aid the practitioner in making informed decisions on a fracture-by-fracture basis. While some favor delaying intervention until swelling resolves, others note the pediatric craniofacial skeleton’s resilience: loose fragments may adhere within 3–4 days of injury.23 Converse advocated prompt repair in the 1960s.35 The authors maintain that if the decision is made to operate, this should be done early so long as the degree of swelling is not prohibitive.
In adults, there are fairly straightforward criteria for operative management of orbital fractures (fracture area greater than 1 cm2 or if over 50% of an orbital wall is involved).36–40
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree