Pathophysiology and Clinical Aspects of Pruritus: Introduction
|
Introduction
Pruritus (itching) is the predominant symptom of skin disease and can best be defined as a sensation that leads to a desire to scratch. All human beings experience this sensation in the course of their lifetime; therefore, it is important to make a distinction between acute itch, which is of a limited period of time ranging from seconds to a week such as the itch related to acute insect bite reaction, and chronic itch, which lasts for months and is the focus of this chapter.1 Chronic itch is a multidimensional phenomenon consisting of sensory, emotional, and cognitive components. In most cases, chronic itch results from interaction of the brain-skin axis. Although itch and pain are separate and distinct sensations, itch has many similarities to pain.2,3 Both itch and pain are unpleasant sensory experiences, follow similar neural pathways, and can severely impair patients’ quality of life. However, the behavioral response patterns differ—pain elicits a reflex withdrawal, whereas itch leads to a scratch reflex.
The limited understanding of itch results from the subjective nature of itching, the absence of specific and sensitive investigational methods to study the neuropathophysiology and molecular basis of itch in humans, the lack of convincing animal models and incomplete knowledge of pharmacologic mediators of pruritus. However, significant progress has been made in the past decade with the discovery of new neural pathways (both histaminergic and nonhistaminergic) as well as novel receptors in humans and animals. The concept that itch is transmitted to the central nervous system (CNS) and processed in the brain should lead to new approaches to antipruritic therapy.4
Epidemiology
Itch is a symptom rather than a specific disease entity; therefore, epidemiologic data for itch are limited. Nevertheless, itch has been found to be the dominant skin complaint among all age groups.5 In a large cross-sectional study in Norway, the prevalence of pruritus was approximately 8% among adults.6 Itch is a primary symptom in a diverse range of skin diseases as well as in systemic diseases. The prevalence of pruritus in different dermatologic and systemic diseases is outlined in Tables 103-1 and 103-2.
Cause | Estimated Prevalence of Pruritus |
|---|---|
Skin disease | |
Atopic dermatitis | 100% |
Contact dermatitis | Unknown |
Poison ivy | Unknown |
Elderly idiopathic xerosis | 30%–60%7 |
Urticaria | 97% |
Extensive psoriasis | 80% |
Pityriasis rosea | Unknown |
Seborrheic dermatitis | Unknown |
Neurodermatitis: lichen simplex chronicus, prurigo nodularis, lichen amyloidosis | Unknown |
Burns | 67%–87%8 |
| Pityriasis rubra pilaris | Unknown |
Dermatitis herpetiformis | Unknown |
Acne | |
Linear immunoglobulin A disease | Unknown |
Bullous pemphigoid | Unknown |
Collagen disease | |
Dermatomyositis | 38% |
Sjögren syndrome | Unknown |
Scleroderma | 45%11 |
Infection | |
Varicella | Unknown |
HIV disease | HIV-associated folliculitis in 25%–50% of HIV patients; nonspecific pruritic eruption in 11%–46% of HIV patients |
Onchocerciasis | 5%–67%12 |
Scabies | 100% (except Norwegian scabies)13 |
Superficial fungal infections | Unknown |
Disease | Estimated Prevalence of Pruritus |
|---|---|
| 25%–85% |
| |
| 20%–25% 100% 4% Unknown |
| |
| 48% Unknown Unknown Unknown 30% Unknown |
| |
| 60% Unknown Unknown 11% 58% |
Etiology and Pathogenesis
Pruritus may originate in the skin or in the CNS. There is no single, definitive classification of pruritus. The International Forum for the Study of Itch (IFSI) has proposed a classification that distinguishes three clinical groups of patients as follows14:
- Group I: Pruritus on diseased (inflamed) skin
- Group II: Pruritus on nondiseased (noninflamed) skin
- Group III: Pruritus presenting with severe chronic secondary scratch lesions, such as prurigo nodularis
The first group includes underlying dermatological diseases, while the second and third group includes patients with systemic diseases including diseases of pregnancy and drug-induced pruritus as well as neuropathic and psychiatric diseases. In some patients, more than one cause may account for pruritus (category “mixed”) while in others no underlying disease can be identified (category “others”).14 It is also important to differentiate acute itch from chronic itch because therapies that provide transient itch relief often do not address the pathological processes underlying chronic itch.1 Moreover, the biologic function of nerve fibers most probably differs in chronic itch than acute itch.
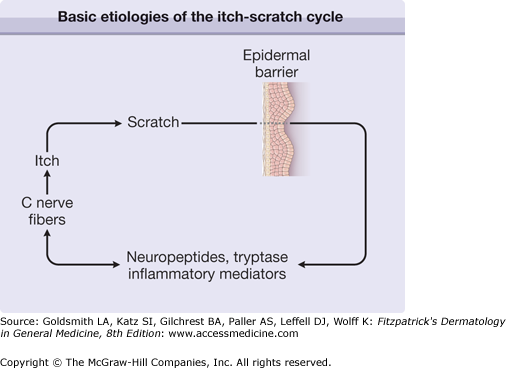
Itch and scratching are interwoven together in both acute and chronic itch conditions. Phylogenetically, itch is probably a mechanism for animals to remove parasites residing in the hairy skin. Scratching is also a behavioral response. A study in humans has shown that repetitive scratching activates the prefrontal cortex in particular, an area of the brain implicated in goal-directed and habit-learning systems.15 It is thus possible that activity induced by scratching in the prefrontal cortex may serve to drive the compulsion to continue scratching and could also account for the highly rewarding aspects of scratching. Furthermore, the hedonic experience of scratching may be associated with release of endogenous opioids. Repetitive scratching in chronic conditions such as atopic dermatitis and psoriasis further damages the skin and causes secretion of neuropeptides and opiates that may further augment the vicious itch-scratch cycle (Fig. 103-1).
Alloknesis is a phenomenon in which a normally innocuous stimulus induces itch. For example, application of a fine brush to an itchy site induces itch. Alloknesis is analogous to the better-known allodynia (defined as pain due to stimuli, which does not normally provoke pain). This type of itch is mediated by mechanical mechanoreceptor units as well as an ongoing activity of afferent C nerve fibers and is considered a central neural sensitization response. Alloknesis is common in chronic atopic dermatitis; sweating or slight mechanical stimulation associated with wearing wool exacerbates itch. The exact role of central sensitization in pruritus associated with specific diseases is unknown.3
The only peripheral tissues from which itch can be evoked are skin, mucous membranes, and cornea. Interestingly, the nerves in the deeper layer of the reticular dermis and subcutaneous fat do not transmit itch and inflammatory skin diseases affecting these areas, such as panniculitis, cause pain but not itch. Removal of the epidermis abolishes perception of pruritus, suggesting that pruritus receptor units are located predominantly within this layer.16 We suspect that the epidermis acts as a receptor for itch, but a specific receptor has not yet been identified. Light microscope and ultrastructural studies of human skin have shown the existence of intraepidermal nerve fibers with “free” nonspecialized nerve endings extending to the stratum granulosum.17 Many epidermal nerve fibers stain positively for neuropeptides implicated in itch transmission. It has recently been shown that Mrgprs, a family of G protein-coupled receptors expressed exclusively in peripheral sensory neurons, function as itch receptors.18
Keratinocytes express a variety of neural mediators and receptors, all of which appear to be involved in the itch sensation.19 Mediators include opioids, proteases, substance P (SP), nerve growth factor (NGF), and neurotrophin 4 whereas receptors include μ- and κ-opioid receptors, proteinase activated receptor-2 (PAR-2), vanilloid receptors, tropomyosin-related kinase A (TRKA), transient receptor potential vanilloid (TRPV) ion channels, gastrin releasing peptide receptor, and cannabinoid receptors 1 and 2. Keratinocytes also have voltage-gated adenosine triphosphate channels and adenosine receptors similar to C nerve fibers. Because these channels have a role in pain,20 these findings suggest that keratinocytes may act as itch receptors.
Significant advances in our understanding of itch neurophysiology have been achieved in the past decade. Microneurography has helped to disprove the historic concept that pruritus and pain are simply responses of the same neurons to mild versus intense stimuli, respectively. Studies using electric field stimulation coupled with microneurography have identified individual histamine-sensitive C nerve fibers that transmit itch.21 The C nerve fibers have exceptionally slow conduction velocity, unusually wide innervation territories, and represent no more than 5% of total C fibers. These neurons are sensitive to pruritogenic and thermal stimuli as well as capsaicin, but not mechanical stimuli. The co-responsiveness of this subset of C neurons to temperature change as well as pruritic stimuli is of interest because raising the temperature of skin lowers the threshold of receptors to pruritic stimuli22 and most pruritic patients complain of aggravation of pruritus in a warm environment. In chronic itch, spontaneous activity in these C fibers occurs.23 In contrast, the vast majority of C fibers are sensitive to mechanical and heat stimuli and are entirely insensitive to histamine.24
The existence of a subset of dedicated itch-transmitting C neurons receives further support from studies of spinal-cord pathways. Itch-transmitting primary afferent C neurons synapse with secondary transmission neurons that cross over to the contralateral spinothalamic tract and ascend to the thalamus. In the cat, microneurography identified lamina 1 neurons in the lateral spinothalamic tract that selectively responded to histamine, suggesting a central dedicated nerve pathway for itch.25 Other C nerve fibers transmit itch as well. Mechanically induced itch is commonly observed clinically; for example, itch associated with contact with wool cannot be explained by histamine-sensitive nerve fibers. Moreover, in patients with chronic pruritus, electrical or painful stimuli can also induce itch.26–28 Oral antihistamines are ineffective in the treatment of most types of itch, suggesting that nonhistamine-mediated fibers also play an important role. A separate nonhistaminergic itch processing pathway activated by cowhage (Mucuna pruriens) was discovered in peripheral nerve fibers of humans as well as in the spinothalamic tract in primates.29,30 The active ingredient inducing itch by cowhage has been found to be a cystine protease which acts through PAR-2 and PAR-4.31 Therefore, two parallel subpopulations of primary afferent C-fibers and spinothalamic tract neurons transmit itch in humans. Both of these pathways are most probably not itch specific since they also transmit burning sensations and respond to the algogen, capsaicin. In addition, gastrin-releasing peptide (GRP) receptor positive neurons were recently found to constitute a dedicated neuronal pathway for itch in the spinal cord of mice.32 The role of these neurons and their interaction with both histaminergic and nonhistaminergic pathways in humans remains to be elucidated.
The perceived sensation of pruritus can vary greatly in quality. Patients may experience sensations of burning or pricking but the neurophysiologic and psychologic correlates of these differences have not yet been elucidated. Information obtained from itch questionnaires based on previously developed pain questionnaires has enabled us to better understand the different characteristics of itch.7,33–35
The central processing of itch has been demonstrated using the neuroimaging techniques of positron emission tomography and functional magnetic resonance imaging in healthy humans and patients with atopic dematitis. In these studies, histamine-induced itch activated various brain areas implicated in sensory and motor function as well as emotion—reflective of the multidimensional aspects of this distressing symptom. A recent study showed that that the central processing of itch in atopic dermatitis is different from that of healthy subjects. The anterior and posterior cingulate cortex as well as the dorsal lateral prefrontal cortex, which are involved in emotions, reward, and memory of negative experiences were significantly activated in patients with atopic eczema but not in healthy subjects.36 The activation of the precuneus, which is located in close proximity to the posterior cingulate cortex seems unique in itch and has rarely been reported in pain imaging. The precuneus is involved in episodic memory retrieval and could be associated with the affective components involved in itch.36,37
![]() Both central and peripheral mediators are important in pruritus. Proinflammatory pharmacologic mediators cause pruritus in inflammatory skin diseases, such as urticaria. Most such mediators also cause other signs of inflammation (pain, erythema due to vasodilation, increased vascular permeability). Several mediators cause pruritus indirectly by evoking release of histamine and other mediators from mast cells (e.g., SP and several opioid peptides) or by potentiating the actions of other mediators [e.g., prostaglandin E1 (PGE1)]. Potentially important peripheral and central mediators of pruritus include histamine, proteases, cathepsins, gastrin-releasing peptide (GRP), opioids, substance P, nerve growth factor (NGF), interleukins (IL), PGs, and noradrenaline.38
Both central and peripheral mediators are important in pruritus. Proinflammatory pharmacologic mediators cause pruritus in inflammatory skin diseases, such as urticaria. Most such mediators also cause other signs of inflammation (pain, erythema due to vasodilation, increased vascular permeability). Several mediators cause pruritus indirectly by evoking release of histamine and other mediators from mast cells (e.g., SP and several opioid peptides) or by potentiating the actions of other mediators [e.g., prostaglandin E1 (PGE1)]. Potentially important peripheral and central mediators of pruritus include histamine, proteases, cathepsins, gastrin-releasing peptide (GRP), opioids, substance P, nerve growth factor (NGF), interleukins (IL), PGs, and noradrenaline.38
![]() Histamine is the archetypal mediator of signs and symptoms of inflammation including pruritus. Histamine is synthesized in the mast cells of the skin and stored in mast cell granules. It is released by these cells in response to a variety of injurious stimuli. Histologically, dermal mast cells and unmyelinated neurons are closely juxtaposed, raising the possibility of a pseudosynaptic relationship and implying a close functional relationship between the immune and nervous systems. Histamine acts to produce itch by way of the H1 receptor and not via H2 receptors.39 Histamine also rapidly causes tachyphylaxis in human skin40; therefore, it is unlikely to be responsible for sustained pruritus. Nevertheless, it is an important mediator in short-lived urticarial wheal-and-flare reactions and causes pruritus after a single intradermal injection. Histamine’s pruritic action can be potentiated by PGE1. Firm evidence for histamine as the main mediator of pruritus is limited to skin diseases such as acute and chronic urticaria, mastocytosis, insect bite reactions, and allergic drug reactions. H1 antihistamines are usually effective in these disorders. The role of histamine in most types of chronic itch is minimal, and antihistamines do not alleviate itch. In atopic dermatitis, histamine has no significant role, and low-sedation H1 antihistamines are ineffective. Other histamine receptors such as H3– and H4-receptors may also play a role in itch, as demonstrated through mouse models.41 In particular, H4-receptor antagonists have been shown to inhibit experimentally induced itch.42,43
Histamine is the archetypal mediator of signs and symptoms of inflammation including pruritus. Histamine is synthesized in the mast cells of the skin and stored in mast cell granules. It is released by these cells in response to a variety of injurious stimuli. Histologically, dermal mast cells and unmyelinated neurons are closely juxtaposed, raising the possibility of a pseudosynaptic relationship and implying a close functional relationship between the immune and nervous systems. Histamine acts to produce itch by way of the H1 receptor and not via H2 receptors.39 Histamine also rapidly causes tachyphylaxis in human skin40; therefore, it is unlikely to be responsible for sustained pruritus. Nevertheless, it is an important mediator in short-lived urticarial wheal-and-flare reactions and causes pruritus after a single intradermal injection. Histamine’s pruritic action can be potentiated by PGE1. Firm evidence for histamine as the main mediator of pruritus is limited to skin diseases such as acute and chronic urticaria, mastocytosis, insect bite reactions, and allergic drug reactions. H1 antihistamines are usually effective in these disorders. The role of histamine in most types of chronic itch is minimal, and antihistamines do not alleviate itch. In atopic dermatitis, histamine has no significant role, and low-sedation H1 antihistamines are ineffective. Other histamine receptors such as H3– and H4-receptors may also play a role in itch, as demonstrated through mouse models.41 In particular, H4-receptor antagonists have been shown to inhibit experimentally induced itch.42,43
![]() Proteinases and their respective receptors (PARs) appear to play an important role in chronic pruritus and in the regulation of cutaneous inflammatory and immune responses. Human dermal mast cells produce two proteases: tryptase and chymase. The proximity of dermal mast cells to afferent C neuron terminals in skin allows a functional relationship by which tryptase induces itch.44 The activated mast cell releases tryptase (along with other mediators, including histamine), which in turn activates the PAR-2 receptor, a G protein-coupled receptor subfamily localized on C fiber terminals. The activated C fibers transmit this information to the CNS, where it may cause the sensation of itch. Additionally, activation leads to local release of neuropeptides including SP. This forms an additional pathway for itch, and represents another example of interaction between the immune and nervous systems.
Proteinases and their respective receptors (PARs) appear to play an important role in chronic pruritus and in the regulation of cutaneous inflammatory and immune responses. Human dermal mast cells produce two proteases: tryptase and chymase. The proximity of dermal mast cells to afferent C neuron terminals in skin allows a functional relationship by which tryptase induces itch.44 The activated mast cell releases tryptase (along with other mediators, including histamine), which in turn activates the PAR-2 receptor, a G protein-coupled receptor subfamily localized on C fiber terminals. The activated C fibers transmit this information to the CNS, where it may cause the sensation of itch. Additionally, activation leads to local release of neuropeptides including SP. This forms an additional pathway for itch, and represents another example of interaction between the immune and nervous systems.
![]() PARs may play a significant role in regulation of cutaneous neurogenic inflammation. In the skin, PAR-2 expressed on sensory neurons is self-activated by peptide ligands exposed after extracellular cleavage by trypsin or mast cell tryptase. PAR-2 agonists have been demonstrated to induce release of calcitonin gene-related peptide and SP, which induce neurogenic inflammation.45 In addition, PAR-2 agonists elicit dose-related scratching in mice supporting a role for PAR-2 in peripheral itch transduction.46,47 Furthermore, studies using dermal microdialysis have shown that levels of tryptase and its receptor (PAR-2) are elevated fourfold in atopic dermatitis.48 PAR-2 is also highly expressed in the epidermis of atopic dermatitis patients. In addition, recent studies have demonstrated the cysteine proteases, cathepsin S and mucunain, to have roles in itch transmitted through nonhistaminergic pathways.31,49 It is noteworthy that proteinase activity can also be found in common allergens50 and staphylococcal skin infections, both of which are known to aggravate atopic dermatitis and itch.
PARs may play a significant role in regulation of cutaneous neurogenic inflammation. In the skin, PAR-2 expressed on sensory neurons is self-activated by peptide ligands exposed after extracellular cleavage by trypsin or mast cell tryptase. PAR-2 agonists have been demonstrated to induce release of calcitonin gene-related peptide and SP, which induce neurogenic inflammation.45 In addition, PAR-2 agonists elicit dose-related scratching in mice supporting a role for PAR-2 in peripheral itch transduction.46,47 Furthermore, studies using dermal microdialysis have shown that levels of tryptase and its receptor (PAR-2) are elevated fourfold in atopic dermatitis.48 PAR-2 is also highly expressed in the epidermis of atopic dermatitis patients. In addition, recent studies have demonstrated the cysteine proteases, cathepsin S and mucunain, to have roles in itch transmitted through nonhistaminergic pathways.31,49 It is noteworthy that proteinase activity can also be found in common allergens50 and staphylococcal skin infections, both of which are known to aggravate atopic dermatitis and itch.
![]() SP is a neuropeptide widely distributed in the periphery and CNS and is thought to intensify itch perception. SP is synthesized in the cell bodies of C neurons and causes vasodilation and increased vascular permeability. Intradermal injection of SP provokes itch as well as characteristics of neurogenic inflammation such as erythema, wheal, and flare.51–53 SP is elevated in atopic dermatitis patients compared to controls and significantly correlates with disease activity.54
SP is a neuropeptide widely distributed in the periphery and CNS and is thought to intensify itch perception. SP is synthesized in the cell bodies of C neurons and causes vasodilation and increased vascular permeability. Intradermal injection of SP provokes itch as well as characteristics of neurogenic inflammation such as erythema, wheal, and flare.51–53 SP is elevated in atopic dermatitis patients compared to controls and significantly correlates with disease activity.54
![]() SP is co-localized with other neurotransmitters, such as serotonin, dopamine, or calcitonin gene-related peptide, and acts as a neuromodulator. Although endogenously released SP does not degranulate mast cells in healthy human skin55 or cause any sensation at physiological concentrations,56 direct communication between nerve fibers and mast cells through SP has been verified.57 High concentrations of SP are required to cause immediate mast cell degranulation; however, low concentrations can specifically activate neurokinin 1 receptors on mast cells, leading to sensitization of these cells and increased production of tumor necrosis factor (TNF-α).58 In turn, TNF-α sensitizes nociceptive nerve endings, which provide further evidence of the extensive crosstalk between nerve and mast cells.
SP is co-localized with other neurotransmitters, such as serotonin, dopamine, or calcitonin gene-related peptide, and acts as a neuromodulator. Although endogenously released SP does not degranulate mast cells in healthy human skin55 or cause any sensation at physiological concentrations,56 direct communication between nerve fibers and mast cells through SP has been verified.57 High concentrations of SP are required to cause immediate mast cell degranulation; however, low concentrations can specifically activate neurokinin 1 receptors on mast cells, leading to sensitization of these cells and increased production of tumor necrosis factor (TNF-α).58 In turn, TNF-α sensitizes nociceptive nerve endings, which provide further evidence of the extensive crosstalk between nerve and mast cells.
![]() Transient receptor potential (TRP) channels are thermal receptors that are expressed in sensory neurons as well as cutaneous keratinocyte and mast cell. They are activated by chemical and thermal stimuli and may have a role in pain and itch. Transient receptor potential vanilloid receptor-1 (TRPV1) is a nonselective cation channel activated by capsaicin, heat and acid, and has recently been found to have a significant role in behavioral scratching responses in mice.59 Interestingly, it was shown TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase.60 Recently, a TRPV3 mutation produced an itchy phenotype in mice suggesting that TRPV3 may also have a role in itch.61 Together, these results indicate that TRPV1 and TRPV3 play a key role in mediating pruritogenic action via histaminergic and nonhistaminergic pathways.
Transient receptor potential (TRP) channels are thermal receptors that are expressed in sensory neurons as well as cutaneous keratinocyte and mast cell. They are activated by chemical and thermal stimuli and may have a role in pain and itch. Transient receptor potential vanilloid receptor-1 (TRPV1) is a nonselective cation channel activated by capsaicin, heat and acid, and has recently been found to have a significant role in behavioral scratching responses in mice.59 Interestingly, it was shown TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase.60 Recently, a TRPV3 mutation produced an itchy phenotype in mice suggesting that TRPV3 may also have a role in itch.61 Together, these results indicate that TRPV1 and TRPV3 play a key role in mediating pruritogenic action via histaminergic and nonhistaminergic pathways.
![]() GRP receptor positive neurons have recently been found to constitute a dedicated neuronal pathway for itch in the spinal cord of mice.32,62 GRP is one of the mammalian bombesin-related peptides. The role of GRPR in the pathogenesis of chronic itch in humans remains to be determined.
GRP receptor positive neurons have recently been found to constitute a dedicated neuronal pathway for itch in the spinal cord of mice.32,62 GRP is one of the mammalian bombesin-related peptides. The role of GRPR in the pathogenesis of chronic itch in humans remains to be determined.
Opioid peptides are potent neurotransmitters and have both a central and a peripheral itch-producing action. Opioids appear to induce itch via two possible mechanisms. First, by degranulation of cutaneous mast cells63 and second, via a direct central and peripheral pruritogenic effect by activating μ-opioid receptors.63–66 Morphine and other endogenous and exogenous μ-opioid receptor agonists are known to cause generalized pruritus.67–70 The concept of central sensitization in chronic pruritus and possible involvement of pruritic mediators located in the CNS is becoming increasingly recognized as important in both cutaneous and systemic diseases. The perception of pruritus is modified by endogenous opiates via central opioid receptors. Generalized pruritus may result from an imbalance between the μ- and κ-opioid systems68,69,71 Activation of μ-opioid receptors stimulates itch perception, whereas κ-opioid-receptor stimulation inhibits μ-receptor effects both centrally and peripherally.68,69,71,72
![]() NGF is the prototypical neurotrophin—a factor that regulates the growth and function of nerve cells. Other members of this family include neurotrophins 3, 4, and 5 and brain-derived neurotrophic factor.73 Increased levels of epidermal NGF correlate with the proliferation of terminal cutaneous nerves and upregulation of neuropeptides. NGF is known to induce sprouting of nerve fibers, sensitization of nerve endings, axonal transport in spinal ganglia (dorsal root ganglion cells), and increased expression of neuropeptides. NGF is required not only for survival and regeneration of sensory neurons but also controls the responsiveness of such neurons to external stimuli.74,75 Increased cutaneous gene expression of NGF in mast cells, keratinocytes, and fibroblasts of atopic dermatitis patients was highly associated with plasma levels of NGF, thus providing data that NGF may contribute significantly to itch in atopic dermatitis.76 Other neurotrophins, such as neurotrophin 4, are upregulated in keratinocytes of atopic dermatitis patients.77
NGF is the prototypical neurotrophin—a factor that regulates the growth and function of nerve cells. Other members of this family include neurotrophins 3, 4, and 5 and brain-derived neurotrophic factor.73 Increased levels of epidermal NGF correlate with the proliferation of terminal cutaneous nerves and upregulation of neuropeptides. NGF is known to induce sprouting of nerve fibers, sensitization of nerve endings, axonal transport in spinal ganglia (dorsal root ganglion cells), and increased expression of neuropeptides. NGF is required not only for survival and regeneration of sensory neurons but also controls the responsiveness of such neurons to external stimuli.74,75 Increased cutaneous gene expression of NGF in mast cells, keratinocytes, and fibroblasts of atopic dermatitis patients was highly associated with plasma levels of NGF, thus providing data that NGF may contribute significantly to itch in atopic dermatitis.76 Other neurotrophins, such as neurotrophin 4, are upregulated in keratinocytes of atopic dermatitis patients.77
![]() There is increasing evidence that IL-31, a Th-2 cytokine, has a role in pruritus. It has been shown that IL-31 and its oncostatin M receptor (OSMR) act as inducers of itch and dermatitis in mice, and that IL-31 is overexpressed in keratinocytes in atopic dermatitis.78 In addition, a missense mutation in the OSMR gene, which encodes oncostatin M-specific receptor b (OSMRb), was found in families affected by familial primary localized cutaneous amyloidosis, a severe localized pruritic disease.79 Furthermore, IL-31 antibody could effectively reduce scratching behavior in an atopic dermatitis-like murine model during the onset of clinical skin manifestations, suggesting the potential therapeutic role of IL-31 antibody in treatment of chronic itch 78,80 In atopic dermatitis, several cytokines induce itch, including IL-2 and IL-6. IL-2, the prototype pruritogenic cytokine, induces itch in atopic dermatitis as well as in cancer patients receiving recombinant IL-2. Inhibition of IL-2 production forms the basis for treatment of atopic dermatitis with cyclosporine and immunomodulators such as tacrolimus and pimecrolimus. Of note, IL-2 and IL-6 have receptors in nerve cells.
There is increasing evidence that IL-31, a Th-2 cytokine, has a role in pruritus. It has been shown that IL-31 and its oncostatin M receptor (OSMR) act as inducers of itch and dermatitis in mice, and that IL-31 is overexpressed in keratinocytes in atopic dermatitis.78 In addition, a missense mutation in the OSMR gene, which encodes oncostatin M-specific receptor b (OSMRb), was found in families affected by familial primary localized cutaneous amyloidosis, a severe localized pruritic disease.79 Furthermore, IL-31 antibody could effectively reduce scratching behavior in an atopic dermatitis-like murine model during the onset of clinical skin manifestations, suggesting the potential therapeutic role of IL-31 antibody in treatment of chronic itch 78,80 In atopic dermatitis, several cytokines induce itch, including IL-2 and IL-6. IL-2, the prototype pruritogenic cytokine, induces itch in atopic dermatitis as well as in cancer patients receiving recombinant IL-2. Inhibition of IL-2 production forms the basis for treatment of atopic dermatitis with cyclosporine and immunomodulators such as tacrolimus and pimecrolimus. Of note, IL-2 and IL-6 have receptors in nerve cells.
![]() PGs enhance histamine-induced itch in the skin.81,82 PGs are generally not themselves pruritogenic when injected into skin; however, intradermal injection of PGE1 enhances pruritus due to histamine subsequently injected into the same site.83 Only pruritic neurons that display lasting activation after histamine are excited by PGE2, and mechanosensitive fibers are unresponsive to both histamine and PGE2.24 Evidence suggests that cyclooxygenase 2 is induced in the spinal cord in response to cutaneous inflammation, and that spinal cord tissue levels of PGE1 and PGE2 correlate with central pruritus. PGE2 has a direct, low-level pruritogenic effect without inducing protein extravasation in healthy and atopic dermatitis patients, suggesting that prostanoids’ peripheral action is not solely via histamine and that they may potentiate pruritus via a nonspecific effect on nerve fibers. Oral administration of aspirin, a cyclooxygenase inhibitor, does not ameliorate pruritus except in polycythemia vera84; however, topical salicylates and topical aspirin often significantly reduces pruritus in patients with chronic localized itch.85 Of note, PGD2 has been shown to play a role in inhibiting pruritus in mice models of atopic-like dermatitis.86,87 In addition, thromboxane (TX) A2 was shown to induce itch-associated responses through the thromboxane prostanoid (TP) receptors located in keratinocytes and skin nerve fibers in mice. This response was abolished by deficiency of the TP receptor and a TP receptor antagonist.88
PGs enhance histamine-induced itch in the skin.81,82 PGs are generally not themselves pruritogenic when injected into skin; however, intradermal injection of PGE1 enhances pruritus due to histamine subsequently injected into the same site.83 Only pruritic neurons that display lasting activation after histamine are excited by PGE2, and mechanosensitive fibers are unresponsive to both histamine and PGE2.24 Evidence suggests that cyclooxygenase 2 is induced in the spinal cord in response to cutaneous inflammation, and that spinal cord tissue levels of PGE1 and PGE2 correlate with central pruritus. PGE2 has a direct, low-level pruritogenic effect without inducing protein extravasation in healthy and atopic dermatitis patients, suggesting that prostanoids’ peripheral action is not solely via histamine and that they may potentiate pruritus via a nonspecific effect on nerve fibers. Oral administration of aspirin, a cyclooxygenase inhibitor, does not ameliorate pruritus except in polycythemia vera84; however, topical salicylates and topical aspirin often significantly reduces pruritus in patients with chronic localized itch.85 Of note, PGD2 has been shown to play a role in inhibiting pruritus in mice models of atopic-like dermatitis.86,87 In addition, thromboxane (TX) A2 was shown to induce itch-associated responses through the thromboxane prostanoid (TP) receptors located in keratinocytes and skin nerve fibers in mice. This response was abolished by deficiency of the TP receptor and a TP receptor antagonist.88
Clinical Findings
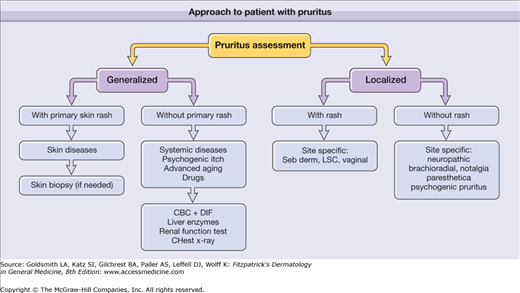
It is of prime importance to determine whether the cause is related to a primary skin disease or systemic disease. Diseases such as skin dryness or scabies may exhibit few primary skin lesions; therefore, careful history and laboratory evaluation can be particularly important. It is important to differentiate between generalized pruritus and localized itch. Careful history, including a full drug history, and physical examination including lymph nodes, are the starting points. The history should take into account the multidimensional nature of itching and should include details of quality, distribution, and timing. Any patient referred with generalized pruritus in the setting of other family members with pruritus should be assumed to have scabies until proved otherwise—skin signs may be clinically inapparent, perhaps confined to a few small nodules on the genitalia. In addition, patients with localized pruritus, especially in a dermatomal distribution, that present with other sensory complaints such as a burning sensation, loss of sensation or increased pain should be evaluated carefully for neuropathic itch. Figure 103-2 is an algorithm showing the approach to a patient with pruritus.
Secondary skin lesions characteristic of pruritus include excoriations, lichenification, and hyper- or hypopigmentation. Lichenification results from continuous rubbing or scratching and consists of well-developed, thickened plaques with marked accentuation of skin creases (see Chapter 14). Post-inflammatory hyperpigmentation or hypopigmentation is common in patients with skin phototypes 4 through 6 (see Chapter 75). Lichenified plaques are most commonly distributed in areas the patient can easily scratch or rub (i.e., nape of neck, below the elbow, ankle, buttock, and genitalia). The butterfly sign consists of normal-appearing skin in the middle of the back outlined by a butterfly pattern of contrasting hyperpigmentation in areas subjected to persistent scratching, resulting from the patient’s inability to reach the middle of the back. Shiny fingernails may result from prolonged rubbing. Prurigo nodules are excoriated papules that form nodules in patients with chronic pruritus (see Chapter 15). In many cases, this type of itch is accompanied by a painful, burning sensation suggestive of a neuropathic component. Prurigo nodules are frequently associated with emotional stress and obsessive-compulsive disorder; however, they can be also be a manifestation of itch in patients with atopic dermatitis or chronic renal failure. Such nodules are usually distributed over extensor aspects of the limbs.
Some pruritic states have specific clinical patterns. Despite severe pruritus, chronic urticaria usually does not show secondary skin lesions associated with scratching. Neuropathic itch in disease entities, such as postherpetic neuralgia, brachioradial pruritus, and notalgia paresthetica, is typically associated with pain and burning sensation. Atopic dermatitis may also be associated with burning sensation after scratching.89
Laboratory tests to be considered in the evaluation of generalized pruritus are outlined in Box 103-1. Secondary laboratory considerations may include stool examination for ova and parasites, screening for hepatitis B or C, plasma protein electrophoresis, and immunoelectrophoresis. A computed tomography scan of the chest and abdomen may be justifiable to help rule out lymphoma. A skin biopsy is not warranted and only useful to exclude clinically inapparent cutaneous mastocytosis, bullous pemphigoid or cutaneous T cell lymphoma.
|
Differential Diagnosis
|
Complications
Pruritus can significantly impair patients’ quality of life and have an effect on prognosis. Patients with chronic pruritus often have difficulty sleeping, difficulty concentrating, decreased sexual desire and sexual function, agitation, and depression.7,90 In addition, eczematous lesions resulting from scratching can become secondarily infected, particularly in patients with atopic dermatitis. Moreover, in a large multinational study in hemodialysis patients, pruritus was associated with a 17% higher mortality risk.91
Clinical Course
Generalized pruritus can wax and wane. Changes in the clinical presentation may be associated with seasonal changes, such as exacerbation of atopic dermatitis in the winter, or changes between dry and humid environments. Pruritus associated with underlying internal disease is often multifactorial, involving both systemic and external factors, including ambient temperature and humidity. Chronic itch associated with skin disease may also include central neural sensitization.28,36
(See Chapter 14.) Pruritus in atopic dermatitis remains a controversial area and the molecular basis of pruritus in atopic dermatitis remains largely unexplained.92 Whether itch precedes skin lesions or vice versa is also an unresolved issue. What is certain is that a vicious itch-scratch cycle (see Fig. 103-1) exists in atopic patients, in which scratch damage enhances pruritus. Itching is particularly acute in response to punctate stimuli such as wool fibers. Alloknesis (see Section “Alloknesis: Itchy Skin”) is a prominent feature of the itch of atopic dermatitis and explains the bouts of intense itching associated with sweating, sudden changes in temperature, dressing, undressing, and direct contact with wool.
A central (neurogenic) component to the itch of atopic dermatitis is suggested by the poor response to low sedation H1 antihistamines.93 The intensity of itch in atopic dermatitis has been related to mental factors, and itch may be induced by cognitive stress, such as anxiety, as well as depression.94–97 Of note, itch intensity and disease severity were significantly correlated with brain activity in the anterior cingulate cortex as well as the insula in atopic dermatitis patients.36 Opioid peptides may serve as central and peripheral mediators because opioid antagonists acting at these levels are effective in some patients.98 Interestingly, there is a significant downregulation of μ-opioid receptor expression in the epidermis of atopic dermatitis patients.99 Nocturnal scratching is a major problem in atopic dermatitis, occurs during superficial sleep, and occupies 10%–20% of the total sleeping time, leading to tiredness and irritability.
Itch in psoriasis is a significant but underrecognized problem in dermatology. Several studies have demonstrated that itch is a principal symptom of psoriasis.100–104 Among psoriasis patients, 77% experience pruritus on a daily basis.90 Dermatologists emphasize observable criteria of psoriasis, such as visible lesions; however, itching frequently occurs in areas of the body where no psoriasis plaques are visible. Scalp itching, in particular, is specific to psoriasis and may require different therapies than pruritus in other areas of the body.103,105
Postherpetic neuralgia commonly has neuropathic pain; and often, associated neuropathic itch in 30%–58% of such patients. Pruritus commonly accompanies both acute zoster and postherpetic neuralgia, particularly lesions affecting the head, face, and neck.106,107
Brachioradial pruritus, a localized pruritus, is becoming increasingly common. Patients, usually fair skinned, affluent, and middle aged, habitually indulge in golf, tennis, sailing, or other leisure outdoor activities in sunny climates.108,109 They develop persistent pruritus of the outer surface of the upper arm, elbow, and forearm, associated with clinical evidence of chronic sun damage and xerosis. The itch is often accompanied by a burning sensation. The itch may gradually become more widespread. The pathophysiology is believed to involve compression of spinal nerve roots in C4–C6 and in rare cases it has been associated with spinal nerve tumors.110 Of note, exposure to UV light has been an eliciting factor.111 (See Chapter 90.)
Notalgia paresthetica is a chronic localized itch, affecting mainly the interscapular area especially the T2–T6 dermatomes, but occasionally with a more widespread distribution, involving the shoulders, back, and upper chest. The sensation perceived by the patient is part itch, part paraesthesia. There are no specific cutaneous signs, apart from those attributed to scratching and rubbing. Amyloid deposition in skin biopsies is a secondary event. The current view on etiology is that it is a neuropathic itch due to nerve entrapment of the posterior rami of spinal nerves arising at T2–T6.112,113
(See Chapter 150.) Pruritus is one of the most distressing symptoms of chronic kidney disease (CKD). It affects 42% of patients on hemodialysis as reported by the Dialysis Outcomes and Practice Pattern Study (DOPPS).91
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree