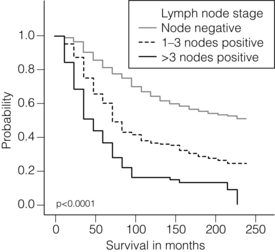
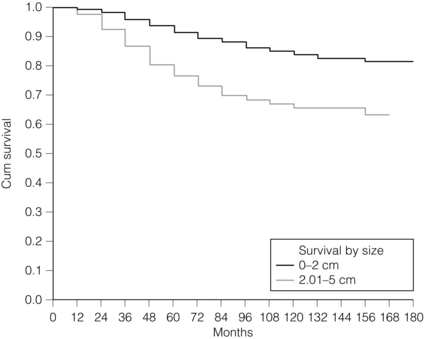
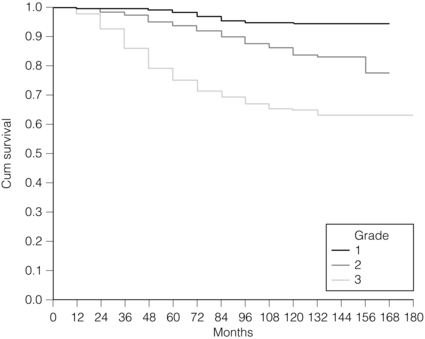
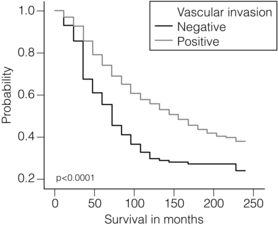
2 1. to separate patients into groups with significantly differing survival probabilities; 2. to separate patients into groups that include a ‘cured’ group and a group with poor survival; 3. to place a sufficient percentage of cases into each group; 4. to be applicable for all operable breast cancers – small, screen detected as well as symptomatic and young age; 5. to be prospectively validated; In this chapter common pathological features of breast cancer are reviewed and their role in guiding patients’ management considered. Involvement of local and regional lymph nodes by metastatic carcinoma is one of the most important prognostic factors in breast cancer. The revised TNM staging system for breast cancer has confirmed that the absolute number of axillary lymph nodes involved in metastatic cancer (‘positive lymph nodes’) is one of the most important prognostic factors in breast cancer.1 Lymph node stage has been used consistently as a guide for therapy. However, lymph node stage is considered as a time-dependent factor; the longer the tumour has been growing the more likely it is that lymph nodes are involved by spread. It has also been reported that, when taken alone, lymph node stage is incapable of defining either a cured group or one with close to 100% mortality from breast cancer. The clinical assessment of nodal status (as in TNM classification) is unreliable.2,3 Palpable nodes may be enlarged because they show benign reactive changes whilst nodes bearing tumour deposits can be impalpable. Although axillary sonography is a moderately sensitive and fairly specific technique in the diagnosis of axillary metastatic involvement,4 histological examination of the lymph nodes from the axilla should be carried out in all patients with primary operable invasive breast cancer. It is well known that patients who have histologically confirmed lymph node involvement have a significantly poorer prognosis than those without nodal metastases. The 10-year survival is reduced from 85% for patients with no nodal involvement to 40% for those with involvement of multiple nodes. Prognosis worsens the greater the number of nodes involved, and the level of nodal involvement can provide useful information; metastasis to the higher level nodes (level II or III) in the axilla and particularly those at the apex (level III) carries a worse prognosis (Fig. 2.1). Optimal management of the axilla is discussed in Chapter 9. A refinement of lymph node sampling is provided by the technique of sentinel lymph node (SLN) biopsy. There have been several studies to examine the validity of this technique. The ALMANAC trial provides level I evidence for UK-based practice.3 Intraoperative frozen sections or imprint cytology of axillary lymph nodes have been advocated by some authors. Conventional frozen sections have a high false-negative rate of between 10% and 30%. More intensive intraoperative assessment with serial sections and immunohistochemistry has been described,8 but is time consuming and labour intensive. Frozen section is best applied to selected cases; for example, if the node is macroscopically abnormal and this is confirmed histologically to be metastatic carcinoma, further axillary surgery can be performed immediately. Some studies have found low false-negative rates of 2–3% with intraoperative imprint cytology,9 but not all have been able to achieve this level of accuracy. Lymph node status can be assessed peroperatively using ultrasound combined with FNA or core biopsy, and this reduces the need for preoperative frozen section and imprint cytology to assess axillary nodes intraoperatively. The detection rate of micrometastases in axillary lymph nodes has been reported to range from 9% to 46%.10 Studies have used serial sectioning with or without immunohistochemical stains to detect micrometastatic foci and these methods have increased detection rates. The European Working Group for Breast Screening Pathology11 has formulated working guidelines for the assessment and pathological work-up of SLNs in breast cancer. From a literature review available at that time, the committee concluded that it was not possible to determine the significance of micrometastasis or isolated tumour cells. They noted that approximately 18% of cases are associated with other nodal (non-sentinel node) metastases. False-negative rates are most often determined via immunohistochemistry (IHC). However, at present it is recommended that an intensive work-up is not justified on a population level. The committee did suggest multilevel assessment and, where resources permit, intraoperative assessment, although the results of Z0011 (see Chapter 7) suggest that some node-positive patients may not require axillary dissection and this brings the whole issue of intraoperative assessment into question. The current view is that IHC should not be used routinely to assess axillary lymph nodes. Tumour size is one of the most powerful predictors of prognostic factor in breast cancer.12,13 The frequency of nodal metastases in patients with tumours < 10 mm is 10–20%,13 and node-negative patients with tumours < 10 mm have a 10-year disease-free survival rate of greater than 90%.14 Tumour size is a time-dependent prognostic factor that depends on the period between tumour development and detection, and on the balance between tumour cell proliferation and death (tumour growth rate). It is well known that the association between increasing tumour size and increasing number of positive lymph nodes and worse outcome is highly statistically significant.13 Therefore, the main aim of population screening with mammography is to detect smaller tumours that are likely to have a better outcome than those that present symptomatically (of a larger size). Patients with smaller tumours have a better long-term survival than those with larger tumours (Fig. 2.2). Estimation of tumour size has assumed particular importance since the introduction of population screening. In most studies the frequency of axillary lymph node metastasis in small < 15 mm (so-called minimally invasive carcinoma (MIC)) invasive carcinomas is 15–20%,15–17 compared with over 40% in tumours measuring 15 mm or more.18 During the prevalent round of breast screening even more favourable results are obtained, with the frequency of axillary lymph node metastasis ranging from 0% to 15%.19–22 The Nottingham Tenovus Primary Breast Cancer Study (NTPBCS) has generated data that suggest the cut-off point of 10 mm is not the best discriminator for MIC. Life-table analysis of survival curves found no difference between tumours measuring up to 9 mm and those measuring 10–14 mm. This indicates that < 15 mm may be a more realistic watershed in defining small, invasive, carcinomas of good prognosis. It is clear that pathological tumour size is a valuable prognostic factor and it has become an important quality assurance measure for breast screening.21,23–26 It is also used in part to judge the ability of radiologists to detect small impalpable invasive carcinomas. Certain histological types of invasive carcinoma carry a favourable prognosis. Tubular, mucinous, invasive cribriform, medullary and tubulolobular types, together with rare tumour types such as adenoid cystic carcinoma, adenomyoepithelioma and low-grade and squamous carcinoma, have all been reported to have a more favourable outcome than invasive carcinoma of no special type (ductal NST). Undoubtedly, assessment of histological type provides prognostic information in breast cancer. However, this effect is relatively small in multivariate analysis27 when compared with the prognostic value of histological grade; histological type may prove to be more useful in increasing our understanding of the biology of breast cancers.28 The Nottingham method (Elston and Ellis) is a modification and improvement of previously existing morphological grading systems and is able to provide greater objectivity of grade.30 • Grade 1 – well differentiated = score 3–5 points. • Grade 2 – moderately differentiated = score 6–7 points. It should be noted that grade is valuable irrespective of histological type. There is a highly significant correlation between tumour grade with long-term prognosis (Fig. 2.3); patients with grade 1 tumours have a 93% chance of surviving 10 years after diagnosis, whereas in patients with grade 3 tumours this is reduced to 70%. It has now been shown conclusively that the Nottingham method, with its more objective criteria, has excellent reproducibility when used by experienced pathologists. The presence of tumour emboli in vascular and lymphatic spaces has emerged as an important prognostic factor. Several studies have now shown that the presence of vascular invasion correlates closely with local and regional lymph node involvement.33,34 It has been suggested that it can provide prognostic information as powerful as lymph node stage. Reproducibility is the limiting factor in its widespread adoption and routine clinical assessment. In a Nottingham study of assessing vascular invasion, the issue of reproducibility was addressed specifically. Of 1704 cases, a subset of 400 cases was examined by two or more pathologists. There was a 77% overall inter-observer agreement on histological features and an 85.8% overall agreement in the classification of vascular invasion. Other studies have reported a similarly high concurrence between pathologists.35,36 The assessment of vascular invasion is subjective but there is good evidence that a high rate of concurrence can be obtained as long as strict criteria are used. Lymphatic and vascular invasion is considered to be a valuable surrogate for lymph node stage in cases where nodes have not been removed for examination. In patients whose axillary nodes are tumour free on histological examination there is a correlation between the presence of lymphovascular invasion (LVI) and early recurrence. Knowledge of the expression of hormone receptors and their subcellular regulatory pathways is one of the classic examples of the use of translational medicine in breast cancer to further diagnosis and treatment of this disease. The degree of ER expression is used to predict an individual’s response to hormone therapy. Both ERs and PRs are steroid receptors that are located in the cell nucleus. Oestrogen and progesterone are considered to diffuse into cells, or be transported to the nucleus. Genes, regulated by steroid receptors, are involved in controlling cell growth, and it is currently believed that these effects are the most relevant to ERs and that ER expression influences the behaviour and treatment of breast cancer.38 Elucidation of downstream effect on genes that are known to be influenced by hormones has led to the inclusion of these ER-regulated genes in high-density oligonucleotide array panels.39 This may better define those pathways that are endocrine responsive and may lead to improved therapies with reduced side-effects and a greater potential for cure.40 In unselected patients, approximately 30% will respond to endocrine therapy. A tumour with both ER and PR expression has a 78% chance of responding to hormone therapy while those cancers that are ER/PR negative respond rarely, if ever.41–43 Due to their close relationship with histological grade, ERs are not of independent prognostic significance.43 There are a multitude of methods available; however, in clinical practice most are based on two distinct strategies. The first is an older method based on ligand-binding methods, i.e. radiolabelled steroid ligand is used to detect the receptor. The second relies on the recognition of the receptor protein by specific antibodies. Several studies examined the correlation between assay and outcome/response and studies have suggested that IHC analyses have a number of advantages.42 IHC is currently the most commonly used method because it can be performed on paraffin-embedded material and allows assessment of the expression specifically in either invasive or in situ cancer. Methods of testing: Several techniques directed at DNA, RNA, protein (and functionally active (activated) as well) or serum can be employed to identify EGFR expression in breast cancer. Of all such methods, IHC is the most practical. The advantages of IHC in EGFR as in HER-2 testing include reproducibility, ease of interpretation and the low cost compared to other techniques. It can also be employed on archival tissue. Protein levels can be quantified by Western analysis or enzyme immunoassay; however, architecture of tissue is lost in these procedures and there may be contamination by normal tissue cells or ductal carcinoma in situ. Serum EGFR levels have been examined in breast carcinoma; however, few data are available.44,45 Due to the paucity of relevant data, it is difficult to draw conclusions on the current value of measuring EGFR in the serum of patients. Prognostic and predictive value: The prognostic value of EGFR in breast cancer has been examined in several large studies. There remains no consensus on its prognostic value. The lack of a clear picture has been attributed to several factors: lack of a standard assay (monoclonal vs. polyclonal), lack of a cut-off for positivity and great variation in study designs (size, follow-up and influence of adjuvant therapy). Klijn et al.46 reviewed data from over 5000 patients where EGFR had been assessed. Findings from this review demonstrated a great heterogeneity of study design, levels of cut-offs for positive and negative, and, not surprisingly, differences in its prognostic value in these various studies. The predictive value of this marker for hormone resistance or responsiveness is better defined. EGFR tumours are considered more resistant to endocrine therapy, and there exists an inverse relationship with EGFR-negative cancers (they are more likely ER positive), being more often sensitive to endocrine manipulation. Though some studies have been able to demonstrate that EGFR status is a predictor of tamoxifen failure and even response rates,50 there are conflicting data from well-designed level II studies51 that have shown no value of EGFR in predicting the efficacy of tamoxifen in high-risk postmenopausal women. Methods of HER-2 testing: Current guidelines for HER-2 testing52 specify the methods that are suitable to detect either the HER-2 protein (by IHC) or gene amplification (using FISH or other in situ methods). Guidelines stress the need for stringent, reproducible and consistent criteria for testing. Immunohistochemistry for HER-2 testing: Among the methods in use for determining HER-2 status, IHC is the most widely used. In studies employing various commercially available antibodies, a wide variety of sensitivity and specificity in fixed paraffin-embedded tissues is seen.53,54 Antigen retrieval techniques are currently not standardised and they introduce the potential for false-positive staining. Nonetheless, IHC possesses many advantages to support its widespread adoption: (i) it allows for the preservation of tissue architecture and so can be used to identify local areas of overexpression within a heterogeneous sample, and can distinguish between HER-2 positivity in in-situ and invasive cancer; (ii) it is applicable to routine patient samples, facilitating use as a diagnostic test, and this allows prospective and retrospective research studies of HER-2 status to be undertaken. Two Food and Drug Administration (FDA)-approved IHC tests for determining HER-2 status are available: HercepTest (DAKO, Carpeteria, CA, USA), based upon a polyclonal antibody; and CB11 (Pathway, Ventana Medical Systems, Tucson, AZ, USA), based upon a monoclonal antibody. The National Comprehensive Cancer Network guidelines55 classify an IHC score of 0 or 1 + as representing HER-2-negative status, 3 + as positive, while 2 + is equivocal. Positive staining is defined as strong, continuous membranous expression of HER-2 in at least 10% of tumour cells. However, a joint report from the American Society of Clinical Oncology (ASCO) and the College of American Pathologists (CAP)52 specified a threshold of > 30% strong circumferential membrane staining for a positive result. If both uniformity and a homogeneous, dark circumferential pattern are seen, resultant cases are likely to be amplified by FISH as well as positive for HER-2 protein expression. The equivocal range for IHC (score 2 +), which may include up to 23% of samples, is defined as complete membrane staining that is either non-uniform or weak in intensity but with obvious circumferential distribution in at least 10% of cells. Equivocal or inconclusive results should be tested by FISH. Consistent with previous guidelines, a negative HER-2 test is defined as either an IHC result of 0 or 1 + for cellular membrane protein expression (no staining or weak, incomplete membrane staining in any proportion of tumour cells), though up to 5% of 1 + on IHC may be FISH positive.
Pathology and biology of breast cancer
Introduction
Traditional factors
Tumour size
Differentiation
Tumour grade
Vascular invasion
Molecular/predictive factors
Oestrogen receptors (ERs)/progesterone receptors (PRs)
Methods of measuring ERs and PRs
Type 1 growth factor receptors
EGFR
HER-2