Pain involves a complex interplay between messages sent from the periphery to the central nervous system and vice versa. Specific pathways play a vital role in carrying these messages, and modulating, or exacerbating their downstream effects. This review describes the anatomy and physiology of pain emphasizing targeted treatment pathways of pain.
Key points
- •
Pain is a multifactorial process that may not always be linked to a stimulus and does not always directly correlate with the severity of injury.
- •
The pain pathways are constantly modulated through physical, biochemical, and psychological interactions.
- •
To optimize the treatment of pain, it is necessary to understand the anatomy and physiology of pain so that targeted therapies may be developed and used.
Introduction
The International Association for the Study of Pain defines pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage”. Pain is often difficult to measure and assess accurately because of its subjectivity where sensation experienced by any one individual has both physical and emotional overtones. Pain, however, is a vital protective sensory phenomenon essential to survival. Patients with leprosy or congenital insensitivity to pain are prone to repeated tissue and organ injuries. Generally, pain is elicited from any stimulus that damages tissue or potentially damages tissue. Pain alerts the individual pathologic affronts on the body and possibly permits avoidance of the offending pathogen or stimulus. However, when the signaling becomes aberrant and chronic, the sensation of pain becomes detrimental to the individual, both physically and psychological. Although in this review the authors describe the physiologic pathways involved in pain, it is important to understand that pain is not always tied to a stimulus. In addition, there may be no direct correlation between the perceived intensity of pain and the severity of tissue damage.
Pain versus nociception
Pain refers to the conscious, subjective experience or perception of a feeling or sensation, which a person calls pain. Nociception is the physiologic activation of neural pathways by stimuli (noxious, thermal, mechanical, or chemical) that are potentially or currently damaging. A stimulus is deemed nociceptive if it results in a behavioral, withdrawal, or escape response. Proprioception is the awareness of oneself or one’s body part relative to their environment. There are many types or descriptions of pain that are descriptive and to some degree may identify sources of ongoing stimulus ( Table 1 ).
| Pain Terminology | Definition |
|---|---|
| Allodynia | Pain due to a stimulus that normally does not provoke pain |
| Causalgia | Syndrome of sustained burning pain, allodynia, and hyperpathia after a traumatic nerve lesions. |
| Dysesthesia | Unpleasant abnormal sensation, spontaneous or evoked |
| Hyperalgesia | Increased pain from a stimulus that normally provokes pain |
| Neuropathic pain | Pain caused by a lesion or disease of somatosensory system |
| Phantom pain | Perception relating to a limb or organ that is not physically part of the body |
| Nociceptive pain | Pain that arises from actual or threatened damage to nonneural tissue; due to activation of nociceptors |
| Acute pain | Pain that lasts less than 3–6 mo and is directly related to tissue damage and resolves as tissues heals |
| Chronic pain | Pain that lasts >3 mo and is primarily mediated by C fibers may have some element of central sensitization |
| Sensitization | Increased responsiveness of nociceptive neurons to their normal input resulting in recruitment of response to normally subthreshold inputs |
Anatomy of pain
In order to understand pain pathways, a brief description of normal anatomy and physiology of the sensory system is required. Afferent sensory nerves send various types of information to the brain. The sensory end organs are made of stimulus accommodating receptors within the skin and tissues ( Fig. 1 ). The various receptors are activated by their stimulus to create an electrical impulse or action potential within the sensory nerve. The action potential is transduced to the nerve cell body within the dorsal root ganglion of the spinal cord. The nerves synapse with a spinal cord nerve that carries the action potential signal to the brain through the spinothalamic and spinoparabrachial tracts. The brain network of signal transduction includes synapses within the parabrachial medulla oblongata, thalamus, amygdala and limbic system, and somatosensory cortex.
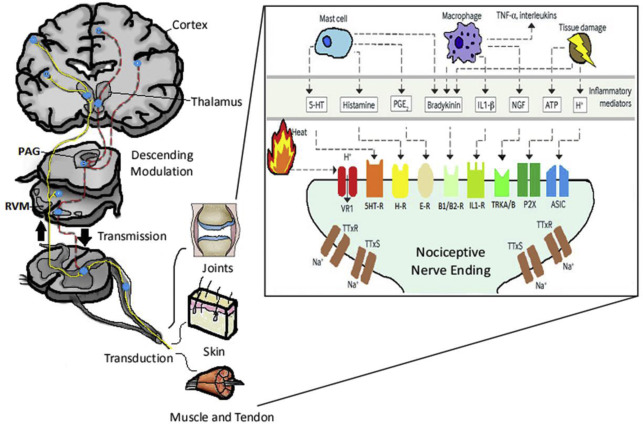
The pathway of pain begins at the periphery with the free nerve endings of primary afferent neurons whose peripheral terminal axons respond to different types of stimuli (mechanical, heat, cold, chemical) , and transduction occurs. The primary afferent fibers are classified based on their conduction velocity and the stimuli that results in their activation ( Table 2 ). A-beta (Aβ) fibers are large-diameter myelinated fibers that are fast conducting (>20 m/s) and activated and produce the sensation of light touch, pressure, or hair movement. A-delta (Aδ) fibers are thinly myelinated fibers that conduct at 2 to 20 m/s. C fibers, the predominant afferent fibers in peripheral nerves, are unmyelinated fibers that conduct at less than 2 m/s. Both Aδ and C fibers may respond to intense heat, cold, mechanical, and chemical stimuli and are subsequently called “polymodal.” Aδ activation elicits an intense, sharp, tingling sensation, whereas C fiber activation results in a dull prolonged burning sensation. Because Aδ fibers conduct at a higher velocity than C fibers, Aδ fibers are believed to convey the “first pain” sensation, whereas C fibers elicit the “second pain” burning sensation. Both Aδ and C fibers are found in skin and other superficial organs, whereas C fibers are the main suppliers of deep structures including muscles and joints. These sensory neurons then synapse in the dorsal horn of the spinal cord in various areas called laminae with second-order neurons. There are 3 types of second-order neurons, including nociceptive specific (NS), wide dynamic range (WDR), and low threshold (LR). NS respond to high-threshold noxious stimuli, WDR respond to sensory stimuli, and LR respond to innocuous stimuli only. These second-order neurons then continue to transmit their signal to the thalamus via the spinothalamic and spinoreticular tracts. The thalamus processes somatosensory information, and neurons within the thalamus project to various regions of the brain including the primary and secondary somatosensory cortices, insula, anterior cingulate cortex, and the prefrontal cortex. The cortex is where the perception of pain (intensity, duration, location) is integrated. The transmission of pain can be modulated throughout this pathway in various locations. Within the spinal cord itself, both excitatory and inhibitory interneurons are present that can modify the pain signal. Within the dorsal root ganglion, the various sensory nerves are modulated by TrkA, TrkB, TrkC receptors, or c-Ret receptors. In fact, there are a multitude of receptors and neurotransmitters throughout the pain pathway that modulate the pain signal and are beyond the scope of this paper (see Fig. 1 ). There are also descending inhibitory tracts involved in reducing pain. The periaqueductal gray (PAG) and nucleus raphe magnus (NRM) are regions in the brainstem that work in concert to block pain transmission.
| Fiber Type | |||
|---|---|---|---|
| Aδ | C | Aβ | |
| Myelinated | Yes | No | Yes |
| Diameter | 1–5 μm | 0.02–1.5 μm | 6–12 μm |
| Conduction speed | 2–20 m/s | < 2 m/s | >20 m/s |
| Thermal sensitivity | Yes/No | Yes/No | No |
| Function | Nociception/Touch | Nociception/Touch | Proprio/mechanoreceptor |
| Modality | Mechanothermal and touch from skin | Polymodal (mechanical, thermal, chemical) | Touch and pressure from skin |
Physiology of pain
There are four processes that occur in the perception of a painful stimulus. The first is transduction. This occurs in the peripheral axons where primary afferent neurons are activated by a noxious stimulus. Receptors found on these axons include vanilloid receptor 1, which responds to heat, capsaicin, and protons, and Mas-related G protein–coupled receptors, which are thought to mediate nociceptive behavior to mechanical stimuli. The next process of the pain pathway is transmission. During this process, pain impulses are transmitted by a 2-fiber system that includes the fast and sharp sensation of the Aδ fibers, and the slower sensation attributed to the C fibers. Both fibers end in the dorsal horn of the spinal cord where Aδ fibers synapse with neurons in laminas I and V and C fibers synapse in laminas I and II. Because of the significant plasticity of the cells located in the dorsal horn, pain impulses can be modified or “gated” in this location. Transmission continues via second-order neurons to the central nervous system via the lateral spinothalamic tract and medial spinothalamic tract. The lateral spinothalamic tract projects to the ventral posterolateral nucleus of the thalamus and informs the brain regarding duration, location, and intensity of pain, whereas the medial spinothalamic tract projects to the medial thalamus and carries the autonomic and unpleasant emotional perception of pain. Third-order neurons in the thalamus subsequently project to specific cortical regions that mediate the perception, localization, and emotional components of pain. Signal modulation is the third process and occurs peripherally, within the spinal cord, and in the brain. Altering neural activity along the pain pathway can result in the suppression or inhibition of pain.
Modulation of pain
The modulation of pain is an endogenous process that is thought to provide a survival advantage. Evidence of this was portrayed in a study by Beecher, an anesthesiologist in World War II, in which they identified soldiers that suffered from severe battle wounds and found that they often experienced little or no pain. This signified that the body possesses an endogenous mechanism that dissociates and modulates (enhances or diminishes) the transmission of pain. Mechanisms responsible for this phenomenon include segmental inhibition, the endogenous opioid system, and the descending inhibitory nerve system. In addition, cognitive and coping strategies also play a role in altering pain perception. Segmental inhibition more commonly known and originally described by Melzack and Wall as the “gate theory” infers that the synapses between afferent neurons that transmit noxious stimuli (Aδ and C fibers) and neurons in the dorsal horn of the spinal cord can be blocked. This occurs when large myelinated nerve fibers (Aβ) that sense touch (nonnoxious stimuli), stimulate the inhibitory nerve in the spinal cord, which in turn inhibits the transmission of the pain signal by suppressing transmission in small unmyelinated C fiber afferents. This explains why rubbing an injury reduces the sensation of pain. The mechanism behind transcutaneous electrical nerve stimulation for pain control is based on this theory. The endogenous opioid system arose when receptors to opium derivatives were found in the central nervous system (PAG, ventral medulla) and spinal cord (lamina I and II). Three groups of endogenous compounds were subsequently identified, and they include enkephalins, endorphins, and dynorphins. These endogenous compounds bind to the opioid receptors in the pain pathway and modulate the pain signal. Finally, the descending inhibitory nerve system controls the transmission of noxious signaling by using the neurotransmitters serotonin and norepinephrine. The limbic system projects to the PAG and medulla before synapsing in the dorsal horn of the spinal cord. Serotonin, synthesized from the NRM, and norepinephrine, synthesized from locus coeruleus, are released to dampen the pain signal in the dorsal horn. These neurotransmitters work to diminish the transmission of pain by (1) direct inhibition of the dorsal horn cells transmitting pain, (2) inhibition of excitatory dorsal horn neurons that work to enhance/exacerbate the transmission of pain, or (3) excitation of inhibitor neurons in the dorsal horn. Collectively, they work to inhibit the transmission of pain through the pain pathways.
Neuropathic pain
Defined as “pain caused by a lesion or disease of the somatosensory system” , has an estimated prevalence of 7% to 9.8% with up to 20% of patients with chronic pain. , Neuropathic pain arises from spontaneous activity or an aberrant response to normal stimulation. It occurs in the presence of an abnormally functioning somatosensory system and can be present in the absence of obvious tissue injury. Unfortunately, it can be refractory to pharmacologic and noninterventional treatment resulting in severe disability and negative impacts on the quality of life. Examples of neuropathic pain include postherpetic neuralgia, postsurgical neuropathy, diabetic neuropathy, peripheral neuropathies, and complex regional pain syndrome. There are many factors that are attributed to neuropathic pain, but an essential component includes injury to the afferent pathway. Other mechanisms include changes in ion channels (quantity and density), central and peripheral sensitization, cortical reorganizations, and disinhibition with cellular and molecular changes. It is also believed that the sympathetic nervous system plays a role in maintaining neuropathic pain. Neuropathic pain is often associated with allodynia, which is a central pain sensitization due to repetitive nonpainful stimulation. Sensitization at the periphery includes the formation of ectopic foci, whereas multiple changes occur centrally, including decreased nonnociceptive input, downregulation of opioid and GABA receptors in the dorsal horn, and death of dorsal horn interneurons in lamina II resulting in “disinhibition” of the pain stimuli. There are also changes in central modulation that result in hyperexcitability of injured nerves, loss of C fibers, as well as increased activity in the sympathetic nervous system. Effective therapies aim to target the receptors and neurotransmitters in these regions and therefore first-line drugs for neuropathic pain include tricyclic antidepressant, serotonin-norepinephrine reuptake inhibitors, gabapentin (best for postherpetic neuralgia and diabetic neuropathy), and pregabalin (shorter time to therapeutic effect and lower effective doses) ( Table 3 ). Second-line therapy includes tramadol, capsaicin patches, and 5% lidocaine patches. Lastly, third-line medications include strong opioids (ie, oxycodone and morphine) and botox (neuropathic pain drugs; see Table 2 ).