This chapter’s focus is the use of pelvic osteotomies for acetabular reorientation for the treatment of symptomatic acetabular dysplasia, including acetabular retroversion.
 The Bernese periacetabular osteotomy (PAO) is a major progression from previously described triple innominate osteotomies (TIO).
The Bernese periacetabular osteotomy (PAO) is a major progression from previously described triple innominate osteotomies (TIO).
The TIO was originally described by Le Coeur30 in 1965. In his technique, the pubis and ischium are osteotomized through a single incision near the symphysis. The ilium is then osteotomized just above the sourcil through a Smith-Petersen approach.
In 1973, Steel46 described his technique for the TIO, in which an incision is made just proximal the gluteal crease and the ischium is divided at the tuberosity. An ilioinguinal approach is then used to divide the ilium, followed by division of the pubis medial to the pectineal tubercle.
In 1981, Tönnis modified Steel’s TIO by changing the location of the ischial cut so that it runs immediately inferior to the acetabulum and ends proximal to the sacrotuberous and sacrospinous ligaments, improving acetabular mobility.51 The ischial osteotomy is performed in the prone position, requiring an intraoperative flip to the supine position, where the pubic and iliac osteotomies are performed similar to those described by Steel.
In 1982, Carlioz introduced a modification to Steel’s technique in which the ischial osteotomy starts just below the acetabulum and runs horizontally, ending between the sacrospinous and sacrotuberous ligaments, leaving the sacrospinous ligament attached to the mobile fragment and the sacrotuberous ligament attached to the stable fragment.4 The benefit of the Carlioz technique is that it does not require an intraoperative flip.
 Periacetabular osteotomy is a general term that describes a series of bony cuts involved in mobilizing the acetabulum from the surrounding innominate bone to allow reorientation but is often used eponymously in reference to Ganz technique for the Bernese PAO.
Periacetabular osteotomy is a general term that describes a series of bony cuts involved in mobilizing the acetabulum from the surrounding innominate bone to allow reorientation but is often used eponymously in reference to Ganz technique for the Bernese PAO.
In 1975, Eppright13 described a barrel-shaped PAO oriented along an anteroposterior axis. This osteotomy allows for increased lateral coverage but limits the amount of achievable anterior coverage.
In 1976, Wagner described three separate types of PAO (types I, II, and III).43
• In a type I PAO, a hemispherical cut is made around the acetabulum down to the obturator foramen using a specifically designed chisel. The loosened acetabular fragment is then redirected to allow anterolateral coverage of the femoral head.
• In a type II PAO, a cut is made similar to type I and autologous bone graft is inserted between the ilium and the acetabular osseous fragment to distalize the acetabular fragment and correct superior subluxation of the femoral head.
• The type III PAO combines a type I osteotomy with a Chiari-like innominate osteotomy to allow realignment and medialization.
The Bernese PAO, originally described by Ganz, uses four or five straight osteotomies to separate the acetabulum from the surrounding innominate bone.15 It has a number of benefits over other techniques, including the following:
• It can be performed through a single incision in the supine position.
• The posterior column of the innominate bone remains intact, greatly improving stability and allowing for immediate postoperative mobilization without a cast or brace.
• The bone cuts are performed very close to the acetabular center of rotation, facilitating correction of the acetabular fragment.
• The Bernese PAO reliably results in medialization of the acetabular center of rotation, improving the biomechanics when compared to previously described techniques.6
• The vascularity of the acetabular fragment is preserved via the inferior gluteal artery, allowing simultaneous hip arthrotomy without concern for devascularization of the mobile fragment.
ANATOMY
 The hip differs from most other joints in the body in that it is deep and nonpalpable from the surface, enforcing the importance of understanding the surface anatomy when planning surgical intervention.
The hip differs from most other joints in the body in that it is deep and nonpalpable from the surface, enforcing the importance of understanding the surface anatomy when planning surgical intervention.
The anterior landmarks consist of the prominent anterior superior iliac spine (ASIS), anterior inferior iliac spine (AIIS), and the medial aspect of the superior pubic rami.
• The ASIS serves as the origin of the sartorius.
• The AIIS serves as the origin of the direct head of the rectus femoris.
• The medial aspect of the superior pubic ramus serves as insertion for the inguinal falx, rectus abdominis, and pyramidalis.
The lateral and posterior landmarks consist of the iliac crest, posterior superior iliac spine (PSIS), and the greater trochanter of the femur.
• The PSIS serves as the attachment of the oblique portion of the posterior sacroiliac ligaments and the multifidus muscle.
• The greater trochanter serves as the attachment of the gluteus medius (superolateral), gluteus minimus (anterior), vastus lateralis (inferolateral), and short external rotators (medial).
• The iliac crest serves as insertion for multiple abdominal muscles, erector spinae, and the tensor fascia lata (TFL).
 During childhood, the articular cartilage of the acetabulum is continuous medially with the triradiate cartilage, lying between the ilium (superiorly), ischium (inferiorly), and pubis (anteriorly).41
During childhood, the articular cartilage of the acetabulum is continuous medially with the triradiate cartilage, lying between the ilium (superiorly), ischium (inferiorly), and pubis (anteriorly).41
Triradiate cartilage closure occurs at the halfway point on the ascending limb of the pubertal growth curve, corresponding to an approximate bone age of 12 years for girls and 14 years for boys.10
The acetabulum is oriented at approximately 48 ± 4 degrees caudal tilt and in 21 ± 5 degrees anteversion.28 It is hemispherical in shape and covers 170 degrees of the femoral head.40
 The hip joint is a highly constrained and inherently stable enarthrodial (ball and socket) diarthrosis (synovial joint), formed by the confluence of the acetabulum with the femoral head.
The hip joint is a highly constrained and inherently stable enarthrodial (ball and socket) diarthrosis (synovial joint), formed by the confluence of the acetabulum with the femoral head.
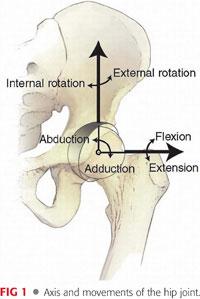
Normal hip range of motion can be highly variable48 (FIG 1).
• Flexion: 110 to 140 degrees
• Extension: 10 to 30 degrees
• Abduction: 40 to 50 degrees
• Adduction: 25 to 30 degrees
• Internal rotation: 30 to 50 degrees
• External rotation: 30 to 60 degrees
The cartilage surface of the acetabulum is horseshoe-shaped with an ovoid depression inferomedially, known as cotyloid fossa, from which the ligamentum teres arises.
• The ligamentum originates in the cotyloid fossa and inserts in the fovea capitis femoris, a depression in the femoral head slightly posteroinferior to center.2
• The ligamentum teres has been shown to contain small vessels, originating from the obturator artery and ranging from only 0.02 to 0.05 cm. These vessels are able to supply only a small portion of the subfoveal femoral head.57
Coverage of the acetabulum is increased by the labrum, a fibrocartilaginous ring running circumferentially around the rim and varying in width from approximately 3 to 8 mm (mean, 5.3 ± 2.6 mm). The labrum increases the surface area of the acetabulum, on average, by more than 25% (36.8 vs. 28.8 cm2) and the volume by more than 30% (41.1 vs. 31.5 cm2).49
• The fibrocartilaginous labrum attaches to the acetabular articular cartilage through a 1- to 2-mm zone of transition, marked by a zone of calcified cartilage with a well-defined tidemark.44
• The labrum is supplied by radial branches of a periacetabular vascular ring derived from the superior and inferior gluteal arteries, with lesser contributions from the medial and lateral circumflex femoral arteries.26
• The transverse acetabular ligament connects the anteroinferior and posteroinferior horns of the labrum as well as the inferior cotyloid fossa.
 The articular capsule consists of strong and dense collagen fibers arranged in a cylindrical sleeve, connecting the margins of the acetabulum to the proximal femur.55 Distinct thickening of the articular capsule forms four reinforcing ligaments:
The articular capsule consists of strong and dense collagen fibers arranged in a cylindrical sleeve, connecting the margins of the acetabulum to the proximal femur.55 Distinct thickening of the articular capsule forms four reinforcing ligaments:
The iliofemoral ligament, also known as the Y-ligament or the ligament of Bigelow, lies anteriorly and has an inverted Y shape. The lateral limb restricts internal and external rotation in extension. The medial limb restricts external rotation in extension.
The pubofemoral ligament resembles a sling covering the inferior and medial aspect of the hip joint capsule and tightens with hip extension and abduction. The pubofemoral ligament’s main restriction is external rotation in extension.
The ischiofemoral ligament lies posteriorly. Its two horizontal bands spiral upward to blend with the zona orbicularis. The ischiofemoral ligament’s major contribution to the stability of the hip is in internal rotation.33
The zona orbicularis is a circumferential ligament surrounding the femoral neck at the lateral edge of the capsule. The fibers of the zona orbicularis are most abundant at the inferoposterior aspect of the capsule and anteriorly blend with the deep surface of the iliofemoral ligament. The zona orbicularis contributes to stability in distraction.23
 The hip joint is surrounded by 23 muscles divided into five groups based on function:
The hip joint is surrounded by 23 muscles divided into five groups based on function:
The flexors are the iliacus, psoas, iliocapsularis, pectineus, rectus femoris (direct and indirect heads), and sartorius muscles.
The extensors are the gluteus maximus, semimembranosus, semitendinosus, biceps femoris (long head), and posterior part of the adductor magnus (ischiopubic ramus origin).
The abductors are the gluteus medius, gluteus minimus, and TFL.
The adductors are the adductor brevis, adductor longus, gracilis, and anterior part of the adductor magnus muscle (ischial tuberosity origin).
The external rotators are the piriformis, quadratus femoris, superior gemellus, inferior gemellus, obturator internus, and obturator externus.
 The acetabulum receives its blood supply from the superior gluteal, inferior gluteal, and obturator arteries.24
The acetabulum receives its blood supply from the superior gluteal, inferior gluteal, and obturator arteries.24
The obturator artery, a branch of the internal iliac artery, passes anteroinferiorly across the inner table of the pelvis, where pubic branches supply the quadrilateral plate. After exiting through the upper border of the obturator foramen, the artery splits into anterior and posterior branches. The posterior branch gives off an acetabular branch, which supplies the acetabulum ascending through the cotyloid fossa.
The largest branch of the internal iliac artery, the superior gluteal artery, heads posterior between the lumbosacral trunk and the first sacral nerve, passes above the superior edge of the piriformis as it exits the greater sciatic notch, then divides into superficial and deep branches. The deep branches descend to supply the superior rim of the acetabulum.
The inferior gluteal artery descends on the sacral plexus before passing below the inferior border of the piriformis, exiting the inferior portion of the greater sciatic notch. Outside the pelvis, a transverse branch runs inferiorly to supply the inferior and posterior region of the acetabulum.
 The corona mortis, an anastomosis between the obturator and external iliac vascular systems traversing cranial to the superior ramus, is present in 83% to 84% of hemipelvis according to two cadaveric studies.9,52 The anastomosis is found, on average, 6.5 cm lateral to the symphysis (range, 3.0 to 9.0 cm). Thirty-four percent to 36% of hemipelvis have an arterial connection, whereas 60% to 70% have a venous connection and 20% to 27.5% have both.
The corona mortis, an anastomosis between the obturator and external iliac vascular systems traversing cranial to the superior ramus, is present in 83% to 84% of hemipelvis according to two cadaveric studies.9,52 The anastomosis is found, on average, 6.5 cm lateral to the symphysis (range, 3.0 to 9.0 cm). Thirty-four percent to 36% of hemipelvis have an arterial connection, whereas 60% to 70% have a venous connection and 20% to 27.5% have both.
 The major pelvic innervations of the lumbar plexus originate from the L1, L2, L3, and L4 roots:
The major pelvic innervations of the lumbar plexus originate from the L1, L2, L3, and L4 roots:
The femoral nerve (L2–L4) courses through the psoas and emerges on the inferolateral aspect of the psoas. It then travels between the psoas and iliacus, behind the iliac fascia until it passes under the inguinal ligament, before bifurcating into anterior and posterior branches.
The lateral femoral cutaneous nerve (LFCN) (L2–L3) emerges from the lateral border of the psoas major and crosses the iliacus muscle toward the ASIS. It then passes under the inguinal ligament medial to the ASIS and over the sartorius in the thigh, where it divides into an anterior and a posterior branch.
• In two cadaveric studies assessing a total of 63 limbs, the LFCN was always found medial to the ASIS and deep to the inguinal ligament.19,54 On average, the LFCN was 3.25 cm medial to the ASIS (range, 0.6 to 9.2 cm).
The obturator nerve (L2–L4) pierces the psoas major, emerging from its medial border near the brim of the true pelvis. It travels posterior to the common iliac artery, then descends lateral to the internal iliac artery and ureter. It then travels along the lateral wall of the lesser pelvis, superior and anterior to the obturator artery, before exiting through the superior edge of the obturator foramen.
 Major pelvic innervations of the lumbosacral plexus originate from the L4, L5, S1, S2, and S3 roots:
Major pelvic innervations of the lumbosacral plexus originate from the L4, L5, S1, S2, and S3 roots:
The sciatic nerve (L4–S3) exits the greater sciatic foramen and most commonly courses anterior (deep) to the piriformis before crossing posterior (superficial) to the superior gemellus, inferior gemellus, and obturator internus. It then travels down the posterior thigh, crossing below the long head of the biceps femoris.
• Approximately 17% of limbs have an anomalous path to the sciatic nerve.45
• Type B anomaly, where the common peroneal branch passes through the piriformis and the common tibial traverses below, is present in 80.9% of anomalous specimens.
• Type C, where the common peroneal branch passes above the piriformis and the common tibial traverses below, is present in 7.6% of anomalous limbs.
• Type D, in which the entire sciatic nerve passes through the piriformis, is present in 3.1% of anomalous gluteal regions.
• Type E, in which the common peroneal branch traverses above the piriformis and the tibial passes through the piriformis, and type F, where the entire sciatic nerve passes above the piriformis, are each present in 0.5% of anomalous hindquarters.
The superior gluteal nerve leaves the pelvis through the greater sciatic notch above the piriformis, accompanied by the superior gluteal artery and the superior gluteal vein. The superior gluteal nerve provides innervation to the gluteus medius, gluteus minimus, and TFL.
The inferior gluteal nerve exits through the greater sciatic notch below the piriformis, accompanied by the inferior gluteal artery and the inferior gluteal vein. The inferior gluteal nerve provides innervation to the gluteus maximus.
PATHOGENESIS
 Developmental dysplasia of the hip (DDH) describes a spectrum of disorders ranging from a shallow acetabulum to a subluxated hip joint to complete dislocation of the hip joint.
Developmental dysplasia of the hip (DDH) describes a spectrum of disorders ranging from a shallow acetabulum to a subluxated hip joint to complete dislocation of the hip joint.
 During embryonic development, the limb buds first appear at approximately 4 weeks’ gestation.56 As early as 6 weeks, an area of densely packed cells between the femoral head and triradiate cartilage mark the area of the future hip joint. By 11 weeks, the basic structures of the hip joint are completely differentiated.
During embryonic development, the limb buds first appear at approximately 4 weeks’ gestation.56 As early as 6 weeks, an area of densely packed cells between the femoral head and triradiate cartilage mark the area of the future hip joint. By 11 weeks, the basic structures of the hip joint are completely differentiated.
 At birth, the femoral head and the acetabulum are primarily cartilaginous. Development of the femoral head and acetabulum requires anatomic reduction as the forces of the femoral head in the acetabulum induce normal development.
At birth, the femoral head and the acetabulum are primarily cartilaginous. Development of the femoral head and acetabulum requires anatomic reduction as the forces of the femoral head in the acetabulum induce normal development.
 DDH occurs in approximately 10 children per 1000, with approximately 1 in 1000 having a frank dislocation; however, this is highly variable based on sex, birth history, family history, and race.20
DDH occurs in approximately 10 children per 1000, with approximately 1 in 1000 having a frank dislocation; however, this is highly variable based on sex, birth history, family history, and race.20
Eighty percent of those diagnosed with DDH are female.59
The left hip is affected in 60% of patients, the right hip in 20%, and both hips in 20%.12 This is thought to be due to the adducted position of the left leg against the mother’s lumbosacral spine in the most common intrauterine position, the left occiput anterior.
The risk of DDH for a child is around 6% when a sibling has DDH, 12% when a parent has DDH, and 36% when both a sibling and a parent have DDH.60
The prevalence is higher in children of Native American or Sami descent and lowest in those of African descent.20
Conditions such as oligohydramnios or breech position predispose patients to DDH.5
 Changes in DDH vary in severity, but in general, patients considered for a PAO in adolescence or early adulthood will have mild symptoms of DDH. The acetabulum is typically shallow, lateralized, anteverted, and deficient along the anterosuperior rim.37 The femoral head is usually small and the neck generally has excessive anteversion with an increased neck–shaft angle. The intramedullary canal of the femur is also generally narrow.
Changes in DDH vary in severity, but in general, patients considered for a PAO in adolescence or early adulthood will have mild symptoms of DDH. The acetabulum is typically shallow, lateralized, anteverted, and deficient along the anterosuperior rim.37 The femoral head is usually small and the neck generally has excessive anteversion with an increased neck–shaft angle. The intramedullary canal of the femur is also generally narrow.
These changes result in a decrease in contact area between the femoral head and acetabulum as well as increased shear at the cartilage interface.
NATURAL HISTORY
 DDH appears in 29% of primary total hip arthroplasties (THA) in people up to the age of 60 years and 7.7% of THA overall.14
DDH appears in 29% of primary total hip arthroplasties (THA) in people up to the age of 60 years and 7.7% of THA overall.14
 In 1939, Wiberg58 published on a series of 18 patients (19 hips) with a center edge angle (CEA) less than 20 degrees whom he followed for 4 to 29 years, all of whom developed osteoarthritis. He showed a linear relationship between decreased CEA and the rate of appearance of arthritis.
In 1939, Wiberg58 published on a series of 18 patients (19 hips) with a center edge angle (CEA) less than 20 degrees whom he followed for 4 to 29 years, all of whom developed osteoarthritis. He showed a linear relationship between decreased CEA and the rate of appearance of arthritis.
In 1983, Cooperman et al8 followed 20 patients (32 hips) (mean age, 43 years) with a CEA of less than 20 degrees for an average of 22 years. All hips developed arthritis; however, the authors found no correlation between the rate of arthritis development and CEA, acetabular angle of Sharp, percentage of the femoral head covered by the acetabulum, acetabular depth, or Tönnis angle. Furthermore, they reevaluated Wiberg’s58 data and found that 7 of the 19 hips were subluxated on initial presentation. When these patients were excluded, they found no correlation between the rate of osteoarthritis development and the aforementioned values.
 Murphy et al,35 in a study of the natural history of DDH, found several criteria that predicted significant arthritis in the hip (Kellgren-Lawrence grade 3 or 4) by the age of 65 years. Poor prognostic factors included the following:
Murphy et al,35 in a study of the natural history of DDH, found several criteria that predicted significant arthritis in the hip (Kellgren-Lawrence grade 3 or 4) by the age of 65 years. Poor prognostic factors included the following:
CEA of less than 16 degrees
Acetabular depth-to-width ratio of less than 38%
Tönnis angle of more than 15 degrees
Uncovering of the femoral head of more than 31%
An acetabulum in which the most cranial point of the dome was at the lateral edge of the acetabulum (peak-to-edge distance)
 In 2005, Jacobsen et al25 reviewed the upright pelvis radiographs of 4151 adults living in Copenhagen as a subanalysis of the Copenhagen City Heart Study. They excluded patients with off-angle radiographs, previous hip fracture, inflammatory arthritis, treatment of childhood hip disorder, or previous hip surgery other than THA, leaving 3859 patients with an average age of 61 years (range, 22 to 93 years) and a CEA ranging from 6 to 67 degrees. The incidence of dysplasia was 3.3% for men and 3.5% for women using a criterion of CEA less than 20 degrees. Risk factors for joint space narrowing (osteoarthritis) included increasing age, in women only, or a CEA of less than 20 degrees.
In 2005, Jacobsen et al25 reviewed the upright pelvis radiographs of 4151 adults living in Copenhagen as a subanalysis of the Copenhagen City Heart Study. They excluded patients with off-angle radiographs, previous hip fracture, inflammatory arthritis, treatment of childhood hip disorder, or previous hip surgery other than THA, leaving 3859 patients with an average age of 61 years (range, 22 to 93 years) and a CEA ranging from 6 to 67 degrees. The incidence of dysplasia was 3.3% for men and 3.5% for women using a criterion of CEA less than 20 degrees. Risk factors for joint space narrowing (osteoarthritis) included increasing age, in women only, or a CEA of less than 20 degrees.
In 2010, Gosvig et al18 reported a very similar study on the same group of 4151 patients with slightly altered exclusion criteria of THA, Legg-Calvé-Perthes disease, childhood hip disease, rheumatoid arthritis, and unreadable radiographs. The overall prevalence of acetabular dysplasia was 4.3% in men and 3.6% in women. Using a cutoff CEA of less than 20 degrees, they found a very strong trend toward osteoarthritis in patients with dysplastic hips; however, this did not reach significance (P = .053). Age was a significant risk factor for arthritis in both sexes (relative risk = 1.02/year, 95% confidence interval [CI] = 1.007 to 1.02).
PATIENT HISTORY AND PHYSICAL FINDINGS
 A thorough history, including a family history of hip problems, is of particular importance.
A thorough history, including a family history of hip problems, is of particular importance.
 Patients with mild to moderate dysplasia may have a painless hip for three or more decades before developing symptoms.
Patients with mild to moderate dysplasia may have a painless hip for three or more decades before developing symptoms.
Most often, patients present between their mid-teens to late 40s, with an average age of 25 to 30 years.
The duration of pain ranges from months to years.
Most patients have seen multiple prior health care providers before an appropriate diagnosis is made, with as many as 15% of the patients having undergone prior surgery for their hip pain.38
 In 1991, Klaue et al27 described acetabular rim syndrome associated with hip dysplasia.
In 1991, Klaue et al27 described acetabular rim syndrome associated with hip dysplasia.
Symptoms include knife-sharp pain in the groin and a sensation of locking of the hip that most often occurred after a period of sitting or sometimes after walking.
Symptoms could be quickly relieved by repositioning and normal walking could be resumed.
Common inciting factors were activities where forced movements of adduction in combination with rotation in either direction occurred, such as pivoting sports.
 Nunley et al37 reviewed the history and physicals of 57 patients and found that the majority (97%) presented with hip pain of insidious onset, localized to the groin in 72% of patients and to the lateral aspect of the hip in 66%.
Nunley et al37 reviewed the history and physicals of 57 patients and found that the majority (97%) presented with hip pain of insidious onset, localized to the groin in 72% of patients and to the lateral aspect of the hip in 66%.
An increase in pain is associated with walking in 81%, running in 80%, standing in 70%, impact activities in 55%, pivoting on the affected side in 45%, and prolonged sitting in 44%.
Eighty percent of patients report catching, clicking, popping, or locking, whereas 48% report a limp and 35% report a limitation in walking distance because of their pain.
 Location and description of the symptoms must be fully delineated as patients may have symptoms related to confounding pathology such as trochanteric bursitis or sacroiliac joint pain, even when the imaging supports a diagnosis of acetabular dysplasia.
Location and description of the symptoms must be fully delineated as patients may have symptoms related to confounding pathology such as trochanteric bursitis or sacroiliac joint pain, even when the imaging supports a diagnosis of acetabular dysplasia.
 During the physical exam, it is important to assess both the affected limb as well as the normal or less affected limb. A general exam of range of motion, strength, tenderness, and visualization of the landmarks and musculature is paramount. Specific tests include the following:
During the physical exam, it is important to assess both the affected limb as well as the normal or less affected limb. A general exam of range of motion, strength, tenderness, and visualization of the landmarks and musculature is paramount. Specific tests include the following:
Gait—a Trendelenburg gait, when the pelvis falls during the stance phase of the affected side, suggests abductor weakness or hip discomfort. A coxalgic, or antalgic, gait is nonspecific and occurs during any cause of hip pain, represented by a shortened swing phase on the affected side. A short limb gait may be present with DDH, usually occurring through pronation of the long leg and supination and a pelvic drop of the short leg. An abnormal gait is seen in 85% of patients, even when walking a short distance.
Leg length (both apparent and true)—a leg length discrepancy of less than 1 cm is normal. Patients with severe DDH often have shortening of the affected limb.
Anterior impingement test—also known as the flexion, adduction, and internal rotation test, the examiner simultaneously flexes (90 to 100 degrees), adducts (10 to 20 degrees), and internally rotates (5 to 20 degrees) the hip. This brings the anterior femoral neck in contact with the anterosuperior rim of the acetabulum, which is the usual site of overload in DDH. A positive test elicits hip pain and reproduces symptoms. Nearly all patients with DDH will test positive for impingement.37
Apprehension test—the hip is extended past neutral and then externally rotated. Pain does not indicate a positive test. The test is positive if the patient complains about the feeling of joint subluxation or instability. A positive test indicates an insufficient coverage of the femoral head.
Trendelenburg test—the examiner observes and palpates the pelvis from behind while the patient performs a single-legged stance. A level pelvis in single-legged stance is normal, and dropping of the contralateral hemipelvis indicates abductor weakness of the symptomatic hip. Abductor weakness is found in more than one-third of patients with DDH.37
Logroll—the lower extremity is rolled side to side at the proximal thigh with the leg in neutral position. The test is positive if it elicits pain in the groin. Logrolling moves only the femoral head in relation to the acetabulum and the surrounding capsule without significant excursion or stress on the surrounding myotendinous structures or nerves, making it the most specific, but not sensitive, test for intracapsular hip pathology.3
IMAGING AND OTHER DIAGNOSTIC STUDIES
 Initial x-ray radiographs should include the standing anteroposterior (AP) pelvis and false-profile views. Optional views include a supine, cross-table lateral, frog-leg lateral, or 90-degree Dunn view. Additionally, a supine AP pelvis with the hip in maximum abduction and internal rotation can be useful to demonstrate hip congruence.
Initial x-ray radiographs should include the standing anteroposterior (AP) pelvis and false-profile views. Optional views include a supine, cross-table lateral, frog-leg lateral, or 90-degree Dunn view. Additionally, a supine AP pelvis with the hip in maximum abduction and internal rotation can be useful to demonstrate hip congruence.
Proper positioning of the patient during radiograph procurement is of paramount importance as improper positioning may result in false-positive findings.
The standing AP image can be taken with the feet in 15 degrees of internal rotation to offset femoral anteversion.
Although the beam will generally be near parallel to the floor, on the AP radiograph, physiologic increase in lumbar lordosis and pelvic tilt must be accounted for so that the tip of the coccyx is within 1 to 3 cm from the superior edge of the pubic symphysis.
The false-profile view, originally described by Lequesne and de Sèze31 in 1961, is a lateral view of the acetabulum taken with the patient standing with the affected hip against the cassette, the pelvis rotated 65 degrees from the AP, and the ipsilateral foot parallel to the cassette.
The Dunn view is an AP view of the hip with the patient supine, the hips and knees flexed at 90 degrees, the legs abducted 15 to 20 degrees from the midline, and the femur in neutral rotation, similar to the position a patient would be on the exam table in stirrups.11
The frog-leg lateral view is obtained with the patient in a standing position. The foot ipsilateral to the affected hip is rested on a step and the affected hip and ipsilateral knee are each flexed approximately 30 degrees. The leg is then externally rotated and abducted.
 The first assessment of any radiograph of a patient being considered for a PAO should evaluate for preexisting osteoarthritis as patients with preexisting degenerative joint disease may not be a candidate for hip preservation surgery, which will be discussed later in this chapter.
The first assessment of any radiograph of a patient being considered for a PAO should evaluate for preexisting osteoarthritis as patients with preexisting degenerative joint disease may not be a candidate for hip preservation surgery, which will be discussed later in this chapter.
 The following should be assessed on the standing AP radiograph:
The following should be assessed on the standing AP radiograph:
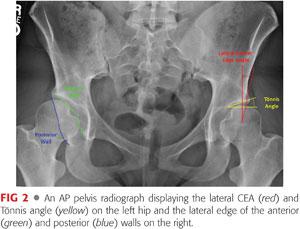
The interrelationship between the anterior and posterior rims of the acetabulum should be evaluated to determine anterior and posterior coverage along with acetabular version. Presence of a crossover sign, where the anterior acetabular rim crosses the posterior rim on the AP radiograph, may represent retroversion of the acetabulum, although this view is sensitive to lumbar lordosis of the patient.42
The visible edge of the posterior wall should descend through the center point of the femoral head or lateral to it. A medial position, or posterior wall sign, may represent posterior dysplasia.
The lateral CEA is traditionally measured using the angle formed by a line drawn perpendicular to a baseline that passes through the center of both femoral heads and a line connecting the center of the femoral head and the edge of the sourcil.58 A CEA of less than 20 degrees or greater than 39 degrees may be indicative of lateral acetabular dysplasia or relative over coverage of the femoral head, respectively.
• Anderson et al1 proposed a more reproducible method for determining the center of the femoral head rather than visual estimation as used by Wiberg.58 In this method, a computer-generated circle is superimposed over each femoral head, ignoring lateral asphericity, and the center of this circle is used as the basis for the lines used for calculation of the CEA.1
The Tönnis angle of inclination, also known as the acetabular index or horizontal toit externe (HTE), is also assessed on the AP radiograph and measures the slope of the weight-bearing region of the acetabulum, or the sourcil.50 The HTE is assessed by the angle formed by a line drawn horizontally through the medial aspect of both sourcils and a line extending from the medial to lateral edges of the sourcil on either side. Normal values range from 0 to 10 degrees, with those higher and lower values indicating relative dysplasia and overcoverage, respectively (FIG 2).
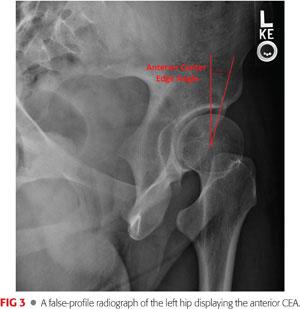
 The false-profile view allows assessment of anterior acetabular coverage through evaluation of the anterior CEA, also known as the vertical-center-anterior (VCA) angle. The VCA angle is measured similar to the CEA using the angle formed by a line drawn perpendicular to a baseline that passes through the center of both femoral heads and a line connecting the center of the femoral head to the anterior edge of the dense shadow of the subchondral bone slightly posterior to the anterior edge of the acetabulum.31 A normal value is that greater than 20 degrees, with lesser values representing anterior dysplasia (FIG 3).
The false-profile view allows assessment of anterior acetabular coverage through evaluation of the anterior CEA, also known as the vertical-center-anterior (VCA) angle. The VCA angle is measured similar to the CEA using the angle formed by a line drawn perpendicular to a baseline that passes through the center of both femoral heads and a line connecting the center of the femoral head to the anterior edge of the dense shadow of the subchondral bone slightly posterior to the anterior edge of the acetabulum.31 A normal value is that greater than 20 degrees, with lesser values representing anterior dysplasia (FIG 3).
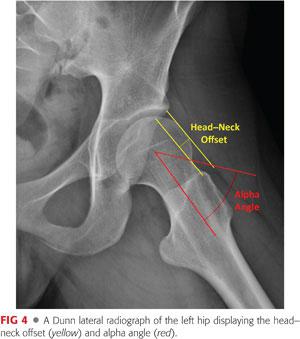
 In hip dysplasia, anterolateral femoral head–neck morphology, which is often characterized by a reduced head–neck offset but not necessarily a true cam deformity, can be evaluated on the frog-leg lateral or 90-degree Dunn view.
In hip dysplasia, anterolateral femoral head–neck morphology, which is often characterized by a reduced head–neck offset but not necessarily a true cam deformity, can be evaluated on the frog-leg lateral or 90-degree Dunn view.
Failure to recognize a decrease of femoral head–neck offset or increased alpha angle preoperatively can cause postoperative anterior femoroacetabular impingement (FAI) through increased anterior acetabular coverage.36 This potential problem should be anticipated preoperatively and managed intraoperatively when present (FIG 4).
 When preoperative radiographs show reason to be concerned for preexisting degeneration of the chondral surface or significant labral pathology, magnetic resonance imaging (MRI) with or without arthrography (MRA) may be useful.
When preoperative radiographs show reason to be concerned for preexisting degeneration of the chondral surface or significant labral pathology, magnetic resonance imaging (MRI) with or without arthrography (MRA) may be useful.
MRA is more valuable in delineating pathologic changes to the labrum but less sensitive in evaluating articular cartilage.
For evaluation of the chondral surfaces, delayed gadolinium-enhanced MRI of cartilage or T1 and T2 relaxation in the rotating frame (T1 rho, T2 rho) are more sensitive.17
 Computed tomography (CT) scans may help the surgeon appreciate acetabular version but are rarely necessary.
Computed tomography (CT) scans may help the surgeon appreciate acetabular version but are rarely necessary.
CT angiogram may be used to assess the integrity of the labrum if an MRA is contraindicated.
DIFFERENTIAL DIAGNOSIS
 Avascular necrosis of the femoral head
Avascular necrosis of the femoral head
 Bursitis
Bursitis
 FAI
FAI
 Hernia (inguinal or femoral)
Hernia (inguinal or femoral)
 Infection (eg, septic arthritis)
Infection (eg, septic arthritis)
 Isolated labral tear
Isolated labral tear
 Lumbar spine pathology (eg, disc herniation)
Lumbar spine pathology (eg, disc herniation)
 Malignancy
Malignancy
 Muscle strain/tear
Muscle strain/tear
 Pelvic pathology (eg, endometriosis or ovarian cysts)
Pelvic pathology (eg, endometriosis or ovarian cysts)
 Sacroiliac joint pathology (eg, ankylosing spondylitis)
Sacroiliac joint pathology (eg, ankylosing spondylitis)
 Femoral neck stress fracture
Femoral neck stress fracture
NONOPERATIVE MANAGEMENT
 Patients who have radiographic findings of dysplasia with no or minimal symptoms should be treated nonsurgically but followed closely and seen in the clinic every 1 to 2 years to evaluate for early signs of joint arthrosis.
Patients who have radiographic findings of dysplasia with no or minimal symptoms should be treated nonsurgically but followed closely and seen in the clinic every 1 to 2 years to evaluate for early signs of joint arthrosis.
 Nonsteroidal anti-inflammatory agents should be used as needed for intermittent symptoms.
Nonsteroidal anti-inflammatory agents should be used as needed for intermittent symptoms.
 High-impact activities such as running should be avoided as these may induce further strain across the hip joint.
High-impact activities such as running should be avoided as these may induce further strain across the hip joint.
 Steroid injections may be employed but should not be used on a regular basis in young individuals.
Steroid injections may be employed but should not be used on a regular basis in young individuals.
 Physical therapy, including hip abductor and core strengthening, may be used with some success.
Physical therapy, including hip abductor and core strengthening, may be used with some success.
SURGICAL MANAGEMENT
 Indications for performance of a PAO include a patient with closed triradiate cartilage and symptomatic acetabular dysplasia without preexisting arthrosis.
Indications for performance of a PAO include a patient with closed triradiate cartilage and symptomatic acetabular dysplasia without preexisting arthrosis.
 Although there is an apparent association between dysplasia and secondary arthrosis, there is potential but no strong evidence that correction of the dysplasia will decrease the development of future arthritis. Thus, a PAO should be a pain-relieving surgery first and a joint-preserving surgery second, not vice versa.
Although there is an apparent association between dysplasia and secondary arthrosis, there is potential but no strong evidence that correction of the dysplasia will decrease the development of future arthritis. Thus, a PAO should be a pain-relieving surgery first and a joint-preserving surgery second, not vice versa.
 Although the lower age limit for PAO is dictated by closure of the triradiate cartilage, the upper age limit is more ambiguous. Any patient with dysplasia without arthrosis may be considered, but in North America, most patients are younger than the age of 40 to 45 years, as those with significant dysplasia will often show signs of significant arthritis by the fifth or sixth decade and may be better served by a THA.16
Although the lower age limit for PAO is dictated by closure of the triradiate cartilage, the upper age limit is more ambiguous. Any patient with dysplasia without arthrosis may be considered, but in North America, most patients are younger than the age of 40 to 45 years, as those with significant dysplasia will often show signs of significant arthritis by the fifth or sixth decade and may be better served by a THA.16
Preoperative Planning
 Using imaging obtained prior to surgery, the surgeon should have a plan regarding the amount of correction needed as well as the direction of correction.
Using imaging obtained prior to surgery, the surgeon should have a plan regarding the amount of correction needed as well as the direction of correction.
 Labs are performed on the day of surgery, including a type and crossmatch for 2 units of packed red blood cells.
Labs are performed on the day of surgery, including a type and crossmatch for 2 units of packed red blood cells.
 Tranexamic acid can be considered to decrease postoperative bleeding.
Tranexamic acid can be considered to decrease postoperative bleeding.
Tranexamic acid is an antifibrinolytic that competitively inhibits the activation of plasminogen to plasmin, thus preventing degradation of blood plasma proteins, most notably fibrin clots.
Although no studies have been performed in patients undergoing PAO, a meta-analysis of the total knee literature has shown that tranexamic acid reduces the amount of blood loss and the number of blood transfusions per patient while showing no difference in the number of deep vein thrombi or pulmonary emboli.61
Dosing can be weight-based (a single dose of 20 mg/kg intravenously preoperatively) or 1 g intravenously at incision and 1 g intravenously at wound closure.
 General endotracheal anesthesia is often combined with a supplemental epidural, which is continued for 48 hours postoperatively.
General endotracheal anesthesia is often combined with a supplemental epidural, which is continued for 48 hours postoperatively.
 A cell saver is used during the surgery because intraoperative blood loss from the bony cuts is variable but often quite rapid, making blood loss significantly high in some patients, even in the hands of an experienced surgeon (FIG 5).
A cell saver is used during the surgery because intraoperative blood loss from the bony cuts is variable but often quite rapid, making blood loss significantly high in some patients, even in the hands of an experienced surgeon (FIG 5).
Positioning
 The patient is placed in the supine position on a radiolucent flat-top table for isolated DDH or on a traction table if intracapsular osteochondroplasty is planned in conjunction. The hip is then prepped and draped in a sterile fashion.
The patient is placed in the supine position on a radiolucent flat-top table for isolated DDH or on a traction table if intracapsular osteochondroplasty is planned in conjunction. The hip is then prepped and draped in a sterile fashion.
Approach
 Five approaches have been described for the Bernese PAO21:
Five approaches have been described for the Bernese PAO21:
Classic Smith-Petersen
Modified Smith-Petersen
Ilioinguinal
Direct anterior
Two-incision
 The modified Smith-Petersen approach is the most common approach and thus will be described in this chapter.
The modified Smith-Petersen approach is the most common approach and thus will be described in this chapter.
TECHNIQUES
 Approach
Approach
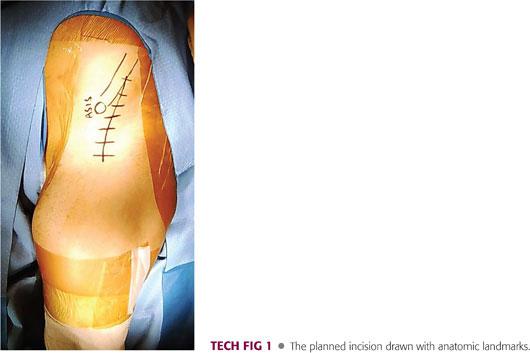
The planned incision begins approximately 3 cm posterior to the ASIS along the iliac crest, extends to a point just lateral to the ASIS, and curves down in line with the lateral border of the sartorius to end approximately 10 cm inferior to the ASIS (TECH FIG 1).
Subcutaneous flaps are raised medially and laterally, aiming to identify the fascia over the TFL muscle belly.
The interval between the sartorius and the TFL is identified distally and traced proximally, incising sharply in line with the fibers while taking great care taken to identify and avoid direct trauma to the LFCN, which courses through the fascia.
The inner table of the ilium is exposed at the proximal portion of the incision by reflecting off the abdominal musculature from the iliac crest to a point just lateral to the ASIS.
The ASIS is osteotomized using an oscillating saw in order to keep the attachment of the sartorius and inguinal ligament in continuity. The ASIS fragment is retracted medially using a Hohmann retractor. Further dissection along the inner iliac wing brings the surgeon to the AIIS and insertion of the direct head of rectus femoris muscle.
Angled retractors are placed for exposure, the rectus femoris is tagged, and both the direct and indirect heads are reflected off the AIIS and anterior acetabulum, respectively, leaving a stump for future repair.
A plane over the anterior hip capsule and under the psoas tendon is developed by reflecting off the iliocapsularis muscle fibers using a Cobb elevator.
 Ischial Osteotomy
Ischial Osteotomy
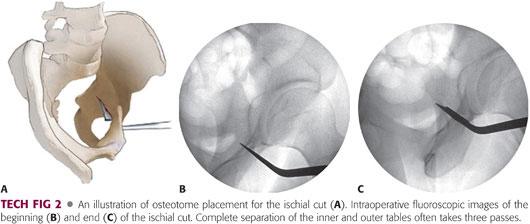
Under two-plane image intensification, an angled Ganz osteotome is placed into the infracotyloid groove between the hip capsule (superiorly), obturator foramen (medially), and the hamstrings musculature (laterally).
The ischial osteotomy most often takes multiple passes. The first two passes of the osteotome are on the inner and outer cortices of the ischium, followed by a central pass to complete the cut.
All passes of the osteotome should be visualized using a two-plane image intensification and should end approximately 1 cm anterior to the posterior cortex of the posterior column.
The cut on the lateral column is often only 2 to 3 cm in depth. Extreme care should be taken with this cut as the sciatic nerve is in close proximity to the lateral aspect of the ischium (TECH FIG 2).
 Pubic Osteotomy
Pubic Osteotomy
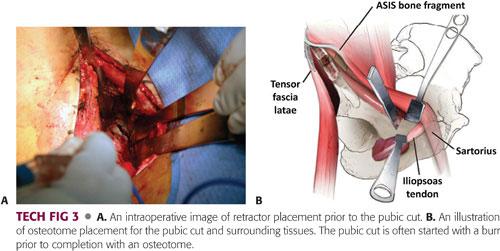
The hip is flexed and the interval along the pubic ramus is further developed through subperiosteal dissection, retracting the psoas tendon and the femoral neurovascular bundle medially with gentle retraction.
Curved rami retractors are placed anterior and posterior to the pubic ramus, subperiosteally, to protect from entering the obturator foramen too deeply and injuring the obturator neurovascular bundle.
A Hohmann retractor can be malleted into the ramus 1 cm medial to the planned osteotomy site for better exposure.
A pencil-tip burr is used to begin the osteotomy of the pubis approximately 1 cm medial to the iliopectineal eminence, and a half-inch curved osteotome is employed to complete the pubic osteotomy (TECH FIG 3).
 Iliac Osteotomy
Iliac Osteotomy
The inner table of the ilium is further exposed through subperiosteal elevation of the iliacus muscle to the level of the greater sciatic notch, where a Hohmann retractor is placed.
The anterior 1 to 2 cm of the TFL origin are reflected from the outer table of the ilium to allow a Hohmann retractor to be placed for protection of the abductor musculature.
An oscillating saw is used to make the transverse, posteriorly directed, supra-acetabular cut across the ilium, starting proximal to the AIIS and stopping 1 cm short of the pelvic brim. Two passes are often necessary, one each on the inner and outer table of the ilium.
The leg should be extended and slightly abducted for the outside cut and slightly flexed for the inside pass (TECH FIG 4A,B).
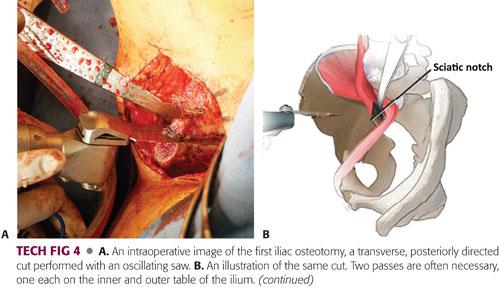
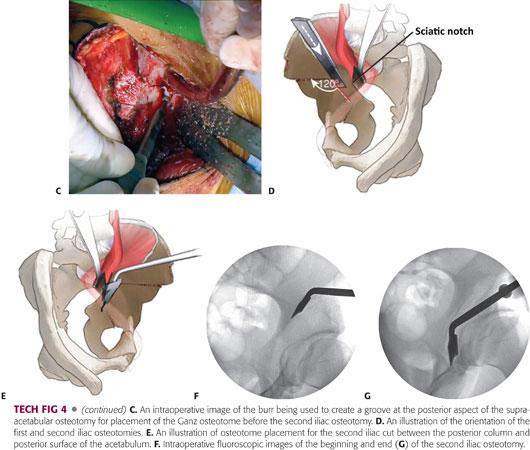
A round-tip burr is used to create a groove angled at approximately 120 degrees, starting from the posterior aspect of the supra-acetabular osteotomy and heading inferiorly toward the ischial spine (TECH FIG 4C,D).
An angled Ganz osteotome is then placed into the grove and passed along the inner table of the quadrilateral plate, under two-plane image intensification, with care taken to stay between the posterior column and posterior surface of the acetabulum. The same osteotome is used to complete the cut through the outer table in a similar fashion. Some surgeons prefer the use of a straight osteotome for the posterior column cut (TECH FIG 4E–G).
 Mobilization, Correction, and Fixation
Mobilization, Correction, and Fixation
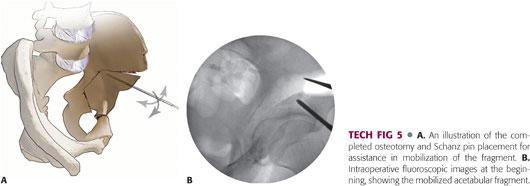
Two Schanz pins are placed into the supra-acetabular region of the mobile fragment for directional control.
A circular motion on the Schanz pins can be used to work free any small remaining bony connections between the mobile fragment and stable pelvis.
If the osteotomies have been completed, the fragment should mobilize relatively easily. Difficulty in mobilizing the fragment should hint at an incomplete osteotomy. In this situation, it is better to revisit the other osteotomies as loosening of the Schanz pins will result in forced mobilization of the fragment with incomplete osteotomies (TECH FIG 5).
Final positioning of the acetabular fragment is unique to each patient. However, general lateral coverage and anteversion are achieved by a combination of internal rotation and slight adduction of the fragment. This maneuver in turn medializes the acetabular fragment and can be verified by the teardrop moving medial to the ilioischial line. After first ensuring a good quality, a nonrotated AP image of the pelvis and hip with two-plane image intensification is used to verify the final position of the acetabulum, with the goal of a horizontal sourcil and lateral CEA of 20 to 30 degrees.
The ideal final position can be confirmed using a nonsterile assistant to overlay a protractor on the image intensifier’s screen.
Once positioned, two small fragment screws are directed from the stable ilium into the superior portion of the osteotomy fragment and a single large fragment screw is directed from the supra-acetabular region of the mobile fragment into the stable ilium. Some surgeons prefer to place all screws antegrade from the stable ilium to the fragment. Final screw placement is checked with image intensification, and stability, along with range of motion, is assessed.
Kirschner wires can be used as needed for provisional fixation.
Flexion and internal rotation should be assessed to ensure repositioning has not caused iatrogenic FAI.
A Horsley bone cutter can be used to remove the excess bone from the superior aspect of the osteotomy fragment and the resected bone can be placed into the space between the ilium and PAO fragment and impacted into place (see FIG 6 in the Postoperative Care section).
At this point, the surgeon may opt to perform an open arthrotomy for any planned intracapsular work.
 Closure
Closure
The rectus femoris is reattached to the AIIS through a drill hole and augmented with another no. 1 nonabsorbable stitch.
A final small fragment screw is used to fix the osteotomized ASIS back into position.
The TFL, subcutaneous, and skin layers are closed with the surgeon’s preference of suture.
A deep and superficial drain may be placed if the surgeon prefers.
PEARLS AND PITFALLS | |
Indications |
|
| |
| |
| |
Contraindications |
|
| |
| |
| |
Preoperative |
|
| |
Technique |
|
| |
| |
| |
| |
| |
| |
Arthrotomy/arthroscopy |
|
| |
POSTOPERATIVE CARE
 Patients should anticipate a 4- to 5-day hospital stay, especially when an epidural is used for postoperative pain control for the first 48 hours.
Patients should anticipate a 4- to 5-day hospital stay, especially when an epidural is used for postoperative pain control for the first 48 hours.
 For anticoagulation, a single dose of low-molecular-weight heparin is given 24 hours postoperatively and then restarted 12 hours after epidural removal until discharge.
For anticoagulation, a single dose of low-molecular-weight heparin is given 24 hours postoperatively and then restarted 12 hours after epidural removal until discharge.
Unless stronger anticoagulation is indicated by a personal or direct family history of deep vein thrombosis or clotting disorder, patients who are ambulating well are discharged on a full-strength (325 mg) aspirin daily for 6 weeks.
 After removal of the epidural, patients begin ambulation with 50% weight bearing on the affected extremity for 6 weeks. After the 6-week follow-up appointment, patients may slowly transition to weight bearing as tolerated.
After removal of the epidural, patients begin ambulation with 50% weight bearing on the affected extremity for 6 weeks. After the 6-week follow-up appointment, patients may slowly transition to weight bearing as tolerated.
 At the 6-week point, patients are prescribed physical therapy for abductor and core strengthening.
At the 6-week point, patients are prescribed physical therapy for abductor and core strengthening.
Patients should expect a mild limp for around 3 months postoperatively.
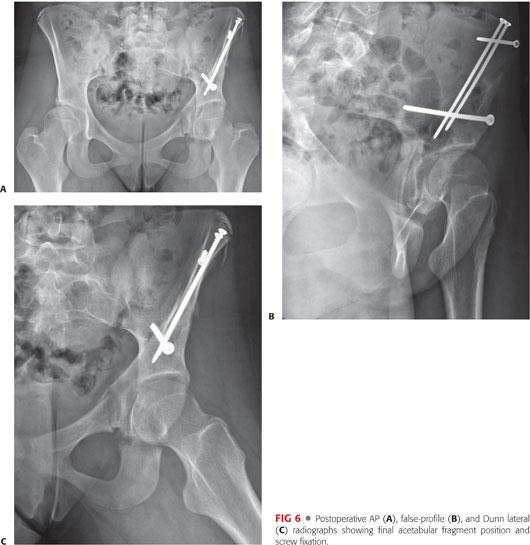
 At both the 6-week and 3-month follow-up appointments, standard postoperative imaging is obtained to evaluate interim bony healing (FIG 6).
At both the 6-week and 3-month follow-up appointments, standard postoperative imaging is obtained to evaluate interim bony healing (FIG 6).
OUTCOMES
 In 2008, Steppacher et al47 reported on a 20-year follow-up of the first 63 patients (75 cases) who underwent Bernese PAO by Ganz. They were able to contact 58 patients, accounting for 68 of the original 75 cases.
In 2008, Steppacher et al47 reported on a 20-year follow-up of the first 63 patients (75 cases) who underwent Bernese PAO by Ganz. They were able to contact 58 patients, accounting for 68 of the original 75 cases.
Forty-one hips (60%) were preserved at 20 years. Hips with Tönnis grade 0 or 1 preoperatively (n = 52) had a survivorship of 75%, whereas those with a preoperative grade 2 or 3 hips (n = 16) had a survivorship of 13%. In those hips that survived, the Tönnis grade increased, on average, 0.7 from baseline.
The average Merle d’Aubigné and Postel score decreased in comparison to the previously reported 10-year value (15.8 vs. 16.7), but it was still slightly higher than patients’ preoperative scores (15.8 vs. 15.2). A score of 18 is excellent, 15 to 17 is good, 13 to 14 is fair, and below 13 is poor.34
 Several studies have reported on midterm follow-up after PAO (average follow-up, 6 to 12 years).
Several studies have reported on midterm follow-up after PAO (average follow-up, 6 to 12 years).
Matheney et al32 described outcomes on 135 hips followed for 9 ± 2.2 years. At final follow-up, 102 hips (76%) remained preserved, with a Western Ontario and McMaster Universities Arthritis Index (WOMAC) pain score of lower than 10. Seventeen underwent THA at an average of 6.1 years after the PAO and 16 had a WOMAC pain score of 10 or higher. Kaplan-Meier analysis, with arthroplasty as the end point, revealed a survival rate of 96% (95% CI, 93% to 99%) at 5 years and 84% (95% CI, 77% to 90%) at 10 years.
Troelsen and colleagues53 reported on 116 PAOs followed for an average of 6.8 years. Kaplan-Meier analysis showed a hip survival rate of 81.6% at 9.2 years. The median physical component score on the Short Form-36 at final follow-up was 48.31, whereas a nonaffected population has a mean score of 50 ± 10. The median pain score on the visual analog scale was 0 at rest and 1 after 15 minutes of normal walking.
Kralj et al29 in 2005 described 26 patients (26 hips) with a mean follow-up of 12 years (range, 7 to 15 years). Four hips (15%) required conversion to THA at a mean of 4.5 years (range, 2 to 7 years). All four hips had preoperative Tönnis grade 2 or 3 osteoarthritis. Thirteen hips (50%) had no radiographic signs of arthritis, but the Tönnis grade increased, on average, 0.8 from preoperative values.
COMPLICATIONS
 A meta-analysis on complications after PAO revealed that 62% of the studies make note of the substantial learning curve and the potential for a higher complication rate during a surgeon’s initial cases.
A meta-analysis on complications after PAO revealed that 62% of the studies make note of the substantial learning curve and the potential for a higher complication rate during a surgeon’s initial cases.
 Reported major complication rates range from 6% to 37%.7
Reported major complication rates range from 6% to 37%.7
The most commonly reported complications included the following:
• Symptomatic heterotopic ossification
• Wound hematomas
• Major nerve (sciatic or femoral) palsies
• Minor nerve (lateral femoral cutaneous) dysfunction
• Intra-articular osteotomies
• Loss of fixation
• Malreduction
• Symptomatic hardware
• Nonunion of at least one osteotomy
Less common complications included the following:
• Deep vein thrombosis
• Pulmonary embolism
• Arterial laceration or thrombosis
• Intra-articular fracture
• Infection
• Major blood loss requiring transfusion
• Femoral head or acetabular osteonecrosis
• Posterior column discontinuity
• Nonunion requiring revision surgery
 In 1999, Hussell et al22 reported on the technical complications in his first 508 consecutive cases. Thirteen of the 508 patients (2.6%) required a salvage arthroplasty due to complications. Eighty-five percent of the complications occurred within the first 50 PAOs performed. Complications consisted of the following:
In 1999, Hussell et al22 reported on the technical complications in his first 508 consecutive cases. Thirteen of the 508 patients (2.6%) required a salvage arthroplasty due to complications. Eighty-five percent of the complications occurred within the first 50 PAOs performed. Complications consisted of the following:
Intra-articular extension (n = 11, 2.2%)
Malreduction (n = 11, 2.2%)
Single osteotomy nonunions (n = 7, 1.4%)
Posterior column fractures (n = 6, 1.2%)
Fragment osteonecrosis (n = 5, 1.0%)
Brooker type IV heterotopic ossification (n = 5, 1.0%)
Sciatic nerve palsy (n = 5, 1.0%)
Failure of fixation (n = 4, 0.8%)
Postoperative subluxation of the femoral head (n = 4, 0.8%)
Femoral nerve palsy (n = 3, 0.6%)
REFERENCES
1. Anderson LA, Gililland J, Pelt C, et al. Center edge angle measurement for hip preservation surgery: technique and caveats. Orthopedics 2011;34(2):86.
2. Bardakos NV, Villar RN. The ligamentum teres of the adult hip. J Bone Joint Surg Br 2009;91(1):8–15.
3. Byrd JW. Evaluation of the hip: history and physical examination. N Am J Sports Phys Ther 2007;2(4):231–240.
4. Carlioz H, Khouri N, Hulin P. Triple juxtacotyloid osteotomy [in French]. Rev Chir Orthop Reparatrice Appar Mot 1982;68(7):497–501.
5. Chan A, McCaul KA, Cundy PJ, et al. Perinatal risk factors for developmental dysplasia of the hip. Arch Dis Child Fetal Neonatal Ed 1997;76(2):F94–F100.
6. Clohisy JC, Barrett SE, Gordon JE, et al. Medial translation of the hip joint center associated with the Bernese periacetabular osteotomy. Iowa Orthop J 2004;24:43–48.
7. Clohisy JC, Schutz AL, St John L, et al. Periacetabular osteotomy: a systematic literature review. Clin Orthop Relat Res 2009;467(8):2041–2052.
8. Cooperman DR, Wallensten R, Stulberg SD. Acetabular dysplasia in the adult. Clin Orthop Relat Res 1983;(175):79–85.
9. Darmanis S, Lewis A, Mansoor A, et al. Corona mortis: an anatomical study with clinical implications in approaches to the pelvis and acetabulum. Clin Anat 2007;20(4):433–439.
10. Dimeglio A. Growth in pediatric orthopaedics. J Pediatr Orthop 2001;21(4):549–555.
11. Dunn DM. Anteversion of the neck of the femur; a method of measurement. J Bone Joint Surg Br 1952;34(2):181–186.
12. Dunn PM. Perinatal observations on the etiology of congenital dislocation of the hip. Clin Orthop Relat Res 1976;(119):11–22.
13. Eppright R. Dial osteotomy of the acetabulum in the treatment of dysplasia of the hip. J Bone Joint Surg Am 1975;57:1172.
14. Furnes O, Lie SA, Espehaug B, et al. Hip disease and the prognosis of total hip replacements. A review of 53,698 primary total hip replacements reported to the Norwegian Arthroplasty Register 1987-99. J Bone Joint Surg Br 2001;83(4):579–586.
15. Ganz R, Klaue K, Vinh TS, et al. A new periacetabular osteotomy for the treatment of hip dysplasias. Technique and preliminary results. Clin Orthop Relat Res 1988;(232):26–36.
16. Garbuz DS, Awwad MA, Duncan CP. Periacetabular osteotomy and total hip arthroplasty in patients older than 40 years. J Arthroplasty 2008;23(7):960–963.
17. Ginnetti J, Erickson J, Peters C. Periacetabular osteotomy: intra-articular work. Instr Course Lect 2012;62:279–286.
18. Gosvig KK, Jacobsen S, Sonne-Holm S, et al. Prevalence of malformations of the hip joint and their relationship to sex, groin pain, and risk of osteoarthritis: a population-based survey. J Bone Joint Surg Am 2010;92(5):1162–1169.
19. Grothaus MC, Holt M, Mekhail AO, et al. Lateral femoral cutaneous nerve: an anatomic study. Clin Orthop Relat Res 2005;(437):164–168.
20. Guille JT, Pizzutillo PD, MacEwen GD. Development dysplasia of the hip from birth to six months. J Am Acad Orthop Surg 2000;8(4):232–242.
21. Hussell JG, Mast JW, Mayo KA, et al. A comparison of different surgical approaches for the periacetabular osteotomy. Clin Orthop Relat Res 1999;(363):64–72.
22. Hussell JG, Rodriguez JA, Ganz R. Technical complications of the Bernese periacetabular osteotomy. Clin Orthop Relat Res 1999;(363):81–92.
23. Ito H, Song Y, Lindsey DP, et al. The proximal hip joint capsule and the zona orbicularis contribute to hip joint stability in distraction. J Orthop Res 2009;27(8):989–995.
24. Itokazu M, Takahashi K, Matsunaga T, et al. A study of the arterial supply of the human acetabulum using a corrosion casting method. Clin Anat 1997;10(2):77–81.
25. Jacobsen S, Sonne-Holm S, Soballe K, et al. Hip dysplasia and osteoarthrosis: a survey of 4151 subjects from the Osteoarthrosis Substudy of the Copenhagen City Heart Study. Acta Orthop 2005;76(2):149–158.
26. Kalhor M, Horowitz K, Beck M, et al. Vascular supply to the acetabular labrum. J Bone Joint Surg Am 2010;92(15):2570–2575.
27. Klaue K, Durnin CW, Ganz R. The acetabular rim syndrome. A clinical presentation of dysplasia of the hip. J Bone Joint Surg Br 1991;73(3):423–439.
28. Kohnlein W, Ganz R, Impellizzeri FM, et al. Acetabular morphology: implications for joint-preserving surgery. Clin Orthop Relat Res 2009;467(3):682–691.
29. Kralj M, Mavcic B, Antolic V, et al. The Bernese periacetabular osteotomy: clinical, radiographic and mechanical 7-15-year follow-up of 26 hips. Acta Orthop 2005;76(6):833–840.
30. Le Coeur P. Correction des défauts d’orientation de l’articulation coxofémorale par ostéotomie de l’isthme iliaque. Rev Chir Orthop 1965;51:211–212.
31. Lequesne M, de Seze S. False profile of the pelvis. A new radiographic incidence for the study of the hip. Its use in dysplasias and different coxopathies. Rev Rhum Mal Osteoartic 1961;28:643–652.
32. Matheney T, Kim YJ, Zurakowski D, et al. Intermediate to long-term results following the Bernese periacetabular osteotomy and predictors of clinical outcome. J Bone Joint Surg Am 2009;91(9):2113–2123. doi:10.2106/JBJS.G.00143.
33. Martin HD, Savage A, Braly BA, et al. The function of the hip capsular ligaments: a quantitative report. Arthroscopy 2008;24(2):188–195.
34. Matta JM. Fractures of the acetabulum: accuracy of reduction and clinical results in patients managed operatively within three weeks after the injury. J Bone Joint Surg Am 1996;78(11):1632–1645.
35. Murphy SB, Ganz R, Muller ME. The prognosis in untreated dysplasia of the hip. A study of radiographic factors that predict the outcome. J Bone Joint Surg Am 1995;77(7):985–989.
36. Myers SR, Eijer H, Ganz R. Anterior femoroacetabular impingement after periacetabular osteotomy. Clin Orthop Relat Res 1999;(363):93–99.
37. Nunley RM, Prather H, Hunt D, et al. Clinical presentation of symptomatic acetabular dysplasia in skeletally mature patients. J Bone Joint Surg Am 2011;93(suppl 2):17–21.
38. Peters CL, Erickson JA, Anderson L, et al. Hip-preserving surgery: understanding complex pathomorphology. J Bone Joint Surg Am 2009;91(suppl 6):42–58.
39. Peters CL, Sierra RJ. Report of breakout session: intraarticular work during periacetabular osteotomy—simultaneous arthrotomy or hip arthroscopy? Clin Orthop Relat Res 2012;470(12):3456–3458.
40. Philippon MJ. The role of arthroscopic thermal capsulorrhaphy in the hip. Clin Sports Med 2001;20(4):817–829.
41. Ponseti IV. Growth and development of the acetabulum in the normal child. Anatomical, histological, and roentgenographic studies. J Bone Joint Surg Am 1978;60(5):575–585.
42. Reynolds D, Lucas J, Klaue K. Retroversion of the acetabulum. A cause of hip pain. J Bone Joint Surg Br 1999;81(2):281–288.
43. Schramm M, Hohmann D, Radespiel-Troger M, et al. The Wagner spherical osteotomy of the acetabulum. Surgical technique. J Bone Joint Surg Am 2004;86(suppl 1):73–80.
44. Seldes RM, Tan V, Hunt J, et al. Anatomy, histologic features, and vascularity of the adult acetabular labrum. Clin Orthop Relat Res 2001;(382):232–240.
45. Smoll NR. Variations of the piriformis and sciatic nerve with clinical consequence: a review. Clin Anat 2010;23(1):8–17.
46. Steel HH. Triple osteotomy of the innominate bone. J Bone Joint Surg Am 1973;55(2):343–350.
47. Steppacher SD, Tannast M, Ganz R, et al. Mean 20-year followup of Bernese periacetabular osteotomy. Clin Orthop Relat Res 2008;466(7):1633–1644.
48. Svenningsen S, Terjesen T, Auflem M, et al. Hip motion related to age and sex. Acta Orthop Scand 1989;60(1):97–100.
49. Tan V, Seldes RM, Katz MA, et al. Contribution of acetabular labrum to articulating surface area and femoral head coverage in adult hip joints: an anatomic study in cadavera. Am J Orthop (Belle Mead NJ) 2001;30(11):809–812.
50. Tönnis D. Normal values of the hip joint for the evaluation of X-rays in children and adults. Clin Orthop Relat Res 1976;119:39–47.
51. Tönnis D, Behrens K, Tscharani F. A modified technique of the triple pelvic osteotomy: early results. J Pediatr Orthop 1981;1(3):241–249.
52. Tornetta P III, Hochwald N, Levine R. Corona mortis. Incidence and location. Clin Orthop Relat Res 1996;(329):97–101.
53. Troelsen A, Elmengaard B, Soballe K. Medium-term outcome of periacetabular osteotomy and predictors of conversion to total hip replacement. J Bone Joint Surg Am 2009;91(9):2169–2179.
54. Uzel M, Akkin SM, Tanyeli E, et al. Relationships of the lateral femoral cutaneous nerve to bony landmarks. Clin Orthop Relat Res 2011;469(9):2605–2611.
55. Wagner FV, Negrao JR, Campos J, et al. Capsular ligaments of the hip: anatomic, histologic, and positional study in cadaveric specimens with MR arthrography. Radiology 2012;263(1):189–198.
56. Watanabe RS. Embryology of the human hip. Clin Orthop Relat Res 1974;(98):8–26.
57. Wertheimer LG, Lopes Sde L. Arterial supply of the femoral head. A combined angiographic and histological study. J Bone Joint Surg Am 1971;53(3):545–556.
58. Wiberg G. Studies on dysplastic acetabula and congenital subluxation of the hip joint, with special reference to the complication of osteo-arthritis. Acta Chir Scand 1939;83(suppl 58):1–135.
59. Wilkinson JA. A post-natal survey for congenital displacement of the hip. J Bone Joint Surg Br 1972;54(1):40–49.
60. Wynne-Davies R. Acetabular dysplasia and familial joint laxity: two etiological factors in congenital dislocation of the hip. A review of 589 patients and their families. J Bone Joint Surg Br 1970;52(4):704–716.
61. Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am 2012;94(13):1153–1159.
< div class='tao-gold-member'>






















