20 Osteoarthritis in the hand and wrist
Synopsis
 Osteoarthritis is characterized by a loss of articular cartilage.
Osteoarthritis is characterized by a loss of articular cartilage.
 The development of osteoarthritis is a dynamic process that represents an imbalance between destruction and repair of the articular cartilage. It can resulft from trauma, such as an intra-articular fracture or a ligamentous injury that results in abnormal load-bearing characteristics or it can be idiopathic without an identifiable cause.
The development of osteoarthritis is a dynamic process that represents an imbalance between destruction and repair of the articular cartilage. It can resulft from trauma, such as an intra-articular fracture or a ligamentous injury that results in abnormal load-bearing characteristics or it can be idiopathic without an identifiable cause.
 Osteoarthritis can affect the entire joint, including the articular cartilage, subchondral bone, ligaments, joint capsule, synovial membrane, and periarticular muscles and tendons.
Osteoarthritis can affect the entire joint, including the articular cartilage, subchondral bone, ligaments, joint capsule, synovial membrane, and periarticular muscles and tendons.
 Patients with osteoarthritis seek treatment because of pain, loss of function, or both. However, there is often a poor correlation between the patient’s symptoms and radiographic findings.
Patients with osteoarthritis seek treatment because of pain, loss of function, or both. However, there is often a poor correlation between the patient’s symptoms and radiographic findings.
 Osteoarthritis is more common in women. In the hand, the DIP joints of the fingers and the CMC joint of the thumb are most commonly affected followed in order by the finger PIP and MP joints.
Osteoarthritis is more common in women. In the hand, the DIP joints of the fingers and the CMC joint of the thumb are most commonly affected followed in order by the finger PIP and MP joints.
 Radiocarpal arthritis is most commonly a result of traumatic injury and follows a regular progression over time as seen in SLAC and SNAC wrist arthritis patterns.
Radiocarpal arthritis is most commonly a result of traumatic injury and follows a regular progression over time as seen in SLAC and SNAC wrist arthritis patterns.
 Treatment of arthritis is directed toward alleviating pain and improving function.
Treatment of arthritis is directed toward alleviating pain and improving function.
 Treatment strategies include operative and nonoperative treatments. Nonoperative treatments include lifestyle modification, hot and cold therapy, splinting, oral or topical anti-inflammatory agents (NSAIDs), and alternative therapies (diet modification, ultrasound, TENS, and acupuncture)
Treatment strategies include operative and nonoperative treatments. Nonoperative treatments include lifestyle modification, hot and cold therapy, splinting, oral or topical anti-inflammatory agents (NSAIDs), and alternative therapies (diet modification, ultrasound, TENS, and acupuncture)
 Surgical management of osteoarthritis includes load altering procedures, joint debridement and/or synovectomy, arthrodesis, and arthroplasty procedures.
Surgical management of osteoarthritis includes load altering procedures, joint debridement and/or synovectomy, arthrodesis, and arthroplasty procedures.
 The appropriate type of operation varies depending on multiple factors including patient age, demands placed on the joint, the requirement for motion to perform one’s activities of daily living or job requirements, patient desires, and the likelihood of success of restoring function and alleviating pain.
The appropriate type of operation varies depending on multiple factors including patient age, demands placed on the joint, the requirement for motion to perform one’s activities of daily living or job requirements, patient desires, and the likelihood of success of restoring function and alleviating pain.
Introduction/epidemiology
• Osteoarthritis (OA) is a heterogeneous disease that includes conditions with different etiology, distribution, heredity, clinical presentation, and progression.
• It is characterized by mechanical and biological events that alter the homeostatic relationship of degradation and repair of the surrounding articular tissues.
• Although all joint tissues are involved, including the subchondral bone, it is damage to the articular cartilage that is the hallmark of the disease.
• Osteoarthritis is the most common rheumatologic disorder in the world. It affects all races, sexes and age groups.
• It is a major cause of adult morbidity. It is the cause of disability in 10% of the population over age 60.1
• In a study from Rotterdam, OA was found in 87% of persons age 55–65.2
• It is estimated that 21% of the population or 46.4 million Americans in 2005 were affected by osteoarthritis.3
• Symptoms of OA may include joint pain, swelling, tenderness, stiffness, and crepitus. OA is typically considered a noninflammatory condition to differentiate it from the inflammatory arthropathies such as rheumatoid and psoriatic arthritis.
• There is no cure for osteoarthritis and no effective method of altering the progression of the disease process.
History
Osteoarthritis is evident across species, with evidence in other mammals, amphibians, certain species of birds and even ancient dinosaurs. The disease is likely as old as man with evidence of its existence in prehistoric man from 2 million years ago.4,5 The disease is considered synonymous with aging.
Basic science/disease process
In order to understand the pathophysiology of osteoarthritis, one must first consider the normal anatomy and biochemistry of the synovial joint. Normal articular cartilage consists of an extensive extracellular matrix composed primarily of proteoglycans, collagens (predominantly type II), and water. While chondrocytes compose only 1% of the volume of the cartilage ECM, they are responsible for maintaining its architecture and composition. The matrix composed primarily of collagen, proteoglycans, proteins and glycoproteins. Cartilage proteoglycans belong to two major classes: large aggregating molecules (aggrecans), and smaller nonaggregating molecules. The aggrecan molecule consists of a central protein core with about 100 glycosaminoglycan (GAG) side branches composed of repeating, negatively-charged disaccharides. Aggrecan molecules form proteoglycan aggregates by linking with a long central hyaluronan molecule. The result is a very large molecule with 105 negatively-charged groups. These groups fill voids in the cartilage framework and create a high osmotic pressure in the framework, providing a stiff construct resistant to compression. Nonaggregating proteoglycans and other matrix proteins serve a variety of functions in the matrix including framework stabilization, regulation of fibrillogenesis, and matrix metabolism through chondrocyte interactions.6 Such molecules include biglycan, decorin, fibromodulin, chondroadherin, cartilage oligomatrix protein, and fibronectin. The interstitial fluid provides an important function in terms of altering the cartilage friction coefficient with changes in pressure (load).7
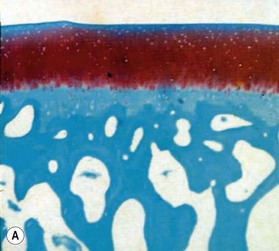
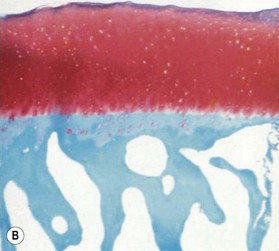
Microscopically, articular cartilage can be divided into four zones based on the cartilage matrix composition and architecture.8 Zone 1 is the most superficial zone and is called the superficial or tangential zone. Here, the chondrocytes are flat and oriented parallel to the articular surface. The collagen is condensed and proteoglycans are sparse. The thin collagen fibers are arranged parallel to the articular surface. This construct resists shear force and has been compared to a “tough skin” that protects the underlying intermediate and deep zones.9 In zone 2, the intermediate zone, the chondrocytes are isolated or in isogenous groups and are surrounded by oblique collagen fibers. This zone is the thickest and is rich in proteoglycans. Proteoglycans are complex macromolecules composed of a protein core with attached glycosaminoglycan chains (chondroitin sulfate and keratan sulfate). The negative charge of the glycosaminoglycans accounts for the hydration and large swelling pressure of cartilage.10 Zone 3 is the radiate layer and includes large, round chondrocytes oriented vertically with intervening radial collagen fibers. Zone 4 is the deepest layer or calcified layer. It lies adjacent to the subchondral bone and resists shear stress between cartilage and bone.9 Between zone 3 and zone 4 is the tidemark (Fig. 20.1). With age, articular cartilage becomes thin and there is relative advancement of the tidemark zone that occurs together with replacement of calcified cartilage with bone.11 The result of this anatomic design is a shiny and slippery surface with a friction coefficient lower than any prosthetic replacement. This remarkable construct is able to withstand millions of loading cycles each year with forces that may reach up to 18 MPa.12
In the healthy, homeostatic state, the chondrocyte responds to the mechanical and biochemical environment by altering the synthesis and degradation its macromolecules. Two groups of enzymes function to degrade the extracellular matrix: matrix metalloproteinases (MMPs) and a disintegrin and metalloproteinase with thrombospondin-like motifs (ADAMTS). MMPs break down collagen and13 ADAMTS functions to break down aggrecan. The main MMP in cartilage is MMP-13 that breaks down type II collagen and the main ADAMTS in cartilage are ADAMTS-4 and ADAMTS-5.14,15 The regulation of matrix degradation and synthesis is poorly understood, but cytokines play an important role in both anabolic and catabolic pathways.8,16,17 The interplay between these cytokines is complex. Anabolic activity seems to be a response of the structural needs of the matrix, as may occur in response to mechanical loading. Cytokines involved in the anabolic pathway include transforming growth factor-beta (TGF-β) and insulin-dependent growth factor I.8 Catabolism within the matrix involves a complex cascade that includes interleukin-1, stromelysin, aggrecanase, and plasmin, in response to stimulation or inhibition by TGF-β, tumor necrosis factor, tissue inhibitors of metalloproteinases, tissue plasminogen activator, plasminogen activator inhibitor, and other molecules.8
Pathophysiology
Osteoarthritis involves all of the tissues around the synovial joint, including the articular cartilage, joint capsule, ligaments, subchondral bone, metaphyseal bone, and the muscles acting across the joint. However, the principle pathological change is that of loss of articular cartilage. Other changes are noted such as subchondral sclerosis, subchondral cyst formation and marginal osteophytes.8,18,19
The earliest microscopic findings of OA are that of fibrillation and fraying of the superficial zone cartilage along with decreased staining for proteoglycans in the superficial and transitional zones and in-growth of blood vessels into the tidemark from subchondral bone (Fig. 20.1B).8 Progression of the disease leads to clefts in the articular surface, fracture of the superficial cartilage and decrease in cartilage thickness. Enzyme activation leads to further destruction of the cartilage leading to complete loss of articular cartilage exposing dense, and eburnated bone.8
Associated with these changes of the cartilage, are alterations in the subchondral bone, specifically an increased density. This increased density can be seen as a sclerotic line on radiograph. These changes may be more pronounced at the joint periphery where the new bone formation may be so exuberant as to produce osteophytes. The exact pathophysiology of osteophyte formation is not known, however it may be related to the release of anabolic cytokines from the matrix that stimulate the abnormal bone and cartilage growth.20,21
The changes involving the joint also affect the periarticular tissues. The synovial membrane becomes inflamed as fragments of cartilage become embedded within them.22 As the joint loses motion due to mechanical factors and pain, the capsule and surrounding ligaments become stiff due to myotendinous contraction and ongoing edema. The lack of motion and use can lead to muscle atrophy.8
Diagnosis
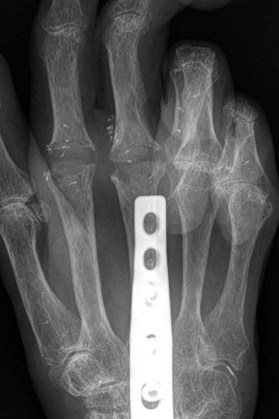
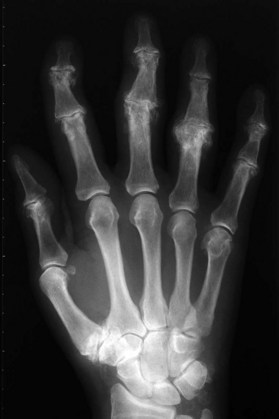
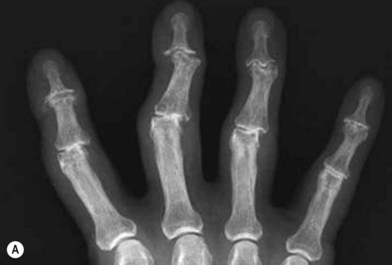
Radiographs are the most helpful test in making the diagnosis. In primary and secondary OA, joint spaces are narrowed radiographically due to a loss of radiolucent articular cartilage. Subchondral bone remodeling can manifest as increased density within the subchondral bone or sclerosis. Osteophytes and loose bodies may also be present (Fig. 20.2).
In 1957, Kellgren and Lawrence described a grading system for the radiographic appearance of OA.23 Findings considered included: (1) the presence of peripheral osteophytes; (2) periarticular ossicles (commonly found in association with DIP and PIP joints); (3) narrowing of joint cartilage and subchondral sclerosis; (4) small pseudocystic areas with sclerotic walls, typically in the subchondral bone, and (5) altered shape of the bone ends. The authors then classified the arthritis into five grades:23
This system has been broadly applied for grading osteoarthritis in various joints; unfortunately the grading system can be ambiguous as there are no specific cut-off criteria between the grades. In a recent analysis by Schiphof and colleagues, the authors note inconsistency in the application of the grading system within multiple cohort studies and have recommended the development of a single validated classification system.24
Management of OA of the fingers
Hand and finger arthritis is one of the most common presentations of primary OA. Its onset is insidious with progressive aesthetic deformity followed by pain and functional limitation. Epidemiological studies of the joint-specific prevalence of hand arthritis consistently show the distal interphalangeal joint to be the site most frequently affected by OA, followed by the thumb CMC joint, and the PIP joint.25 These studies typically defined OA based on the radiographic features described by Kellgren and Lawrence, which include the presence of osteophytes, joint space narrowing, subchondral sclerosis and subchondral cysts.23
Distal interphalangeal joint (DIP) arthritis
Diagnosis
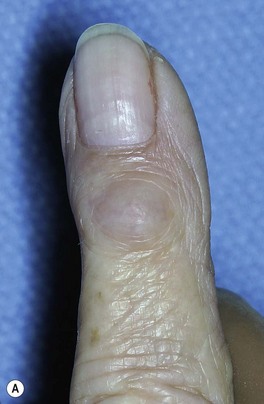
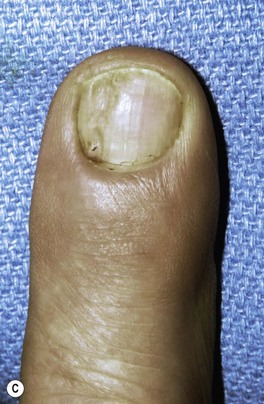
Osteoarthritis of the distal phalangeal (DIP) joint is common and affects females more frequently than males. Clinically, patients present with enlarged joints and hard, knobby protuberances overlying the DIP joints of multiple fingers. These, so called, Heberden’s nodes are pathognomonic for the condition and are attributed to osteophyte formation with overlying soft tissue thickening. These should be differentiated from a mucous cyst. A mucous cyst is a dorsal synovial cyst, often limited to a single digit. They are often associated with lateral deviation of the distal phalanx, and a limited range of motion (Fig. 20.3).
Indications for surgery
The surgical options for treatment of severe DIP arthritis are essentially limited to arthrodesis, which is well tolerated in the DIP position. Although implant arthroplasty is technically feasible, it is not commonly performed because of concerns of long-term joint instability26–30
Biomechanical effects of DIP fusion
The normal range of motion at the DIP joint is 0–60°; however only 15% of digital flexion occurs at the DIP and the joint only contributes 3% of the overall flexion arc of the whole finger.31 Thus of all finger joint fusions, DIP arthrodesis confers the least detrimental impact to hand motion, and is the well tolerated. One study of splint-simulated DIP fusion found a 20% reduction in grip strength which was attributed to the quadriga effect, as a result of the limited excursion of the FDP tendon in the fused digit.32 Significant loss of grip strength has not been shown in clinical studies.
DIP arthrodesis
Techniques
A multitude of fixation techniques have been described,33 including interosseous wiring,34 percutaneous pinning, tension band wiring,35,36 bio-resorbable pinning,37 plating,38 oblique lag screw fixation,39 and axial compression screw fixation.33,40 Regardless of the technique chosen, the joint is prepared in a similar manner and the requirements for a successful outcome are: (1) full apposition of the cancellous bone surfaces; (2) preservation of distal phalanx bone stock to allow for hardware purchase; (3) fusion in approximately 5–10° of flexion if possible, and (4) stable fixation.
With the base of the distal phalanx and the condyles of the middle phalanx exposed, a rongeur is used to remove dorsal and lateral osteophytes. The articular surfaces are decorticated until cancellous bone is visualized. Alternatively, osteotomies can be created with a small oscillating saw to achieve precise apposition of the cancellous bone. If power saws or burrs are to be utilized, copious irrigation should be maintained throughout the cutting process to avoid the risk of thermally induced osteonecrosis which can elevate the risk of nonunion postoperatively.41
Interosseous wiring
Interosseous wiring involves securing the distal and middle phalanx together by means of one or two dental wires passed through bone tunnels. Interosseous wiring can be used to achieve some compression of the joint and is usually used in conjunction with K-wires for greater stability. Zavitsanos et al. described a technique which allows the K-wires to be completely buried, minimizing the risk of infection.34
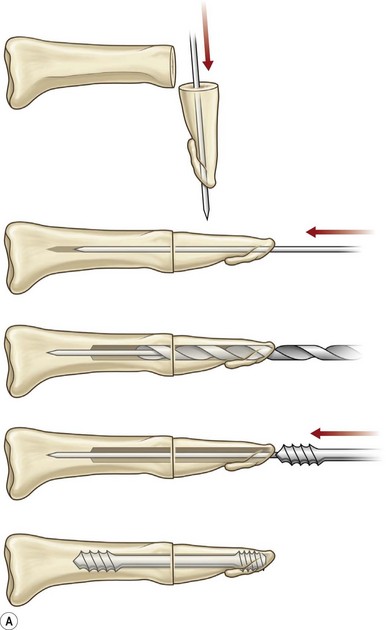
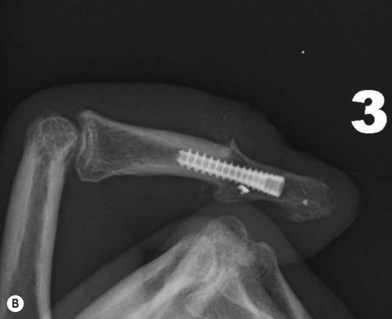
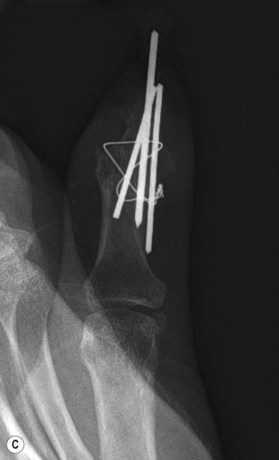
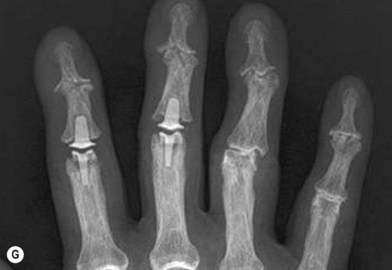
Axial compression screw
A single axial compression screw (Fig. 20.4) can be used to reliably achieve DIP joint fusion. A traditional lag screw technique can be used but will result in prominent hardware at the fingertip and is discouraged. Buried headless compression screws are preferred. The technique for the use of a threaded headless compression screw initially involves preparation of the joint surfaces followed by the placement of an antegrade axial K-wire through the distal phalanx exiting at the hyponychium. The bone surfaces are then coapted in the position of fusion and the same wire is drilled retrograde back into the middle phalanx. The position of the wire and arthrodesis are checked fluoroscopically, and a cannulated drill is used prepare the channel for screw placement. The appropriate screw size is determined through fluoroscopic measurement, and a cannulated, fully threaded; self-tapping screw is inserted in a retrograde fashion to complete the fusion (Fig. 20.4).
The screw should be long enough to engage the cortical isthmus of the middle phalanx. Occasionally, the intramedullary canal of the middle phalanx is too wide for the thread to engage the endosteal cortex even at the isthmus (e.g., thumb), and the chances of a stable construct are reduced. Additionally, specific attention should be paid to the anteroposterior (narrowest) diameter of the distal phalanx to ensure that the screw thread diameter is not greater than this measurement, otherwise dorsal fracturing and nail bed deformity can occur.42
The major advantages of this technique over other methods of DIP fusion are buried hardware and stable fixation. Immobilization time with the use of compression screws has been shown to be significantly shorter when compared to other techniques for DIP fusion. Patients may often be splinted for only one to 3 weeks, allowing patients to return to work earlier.43 One disadvantage of this technique is the difficulty in achieving the optimal prehensile position at the DIP as the joint is typically fused straight due to the conical shape of the screw. Other reported complications have included a single case of skin necrosis of the finger tip requiring secondary amputation;44 this was likely due to unrecognized prominent hardware.
Complications of DIP fusion
Infection
Traditional fixation techniques for small joint arthrodesis include interosseous wiring, crossed K-wires or a combination of these techniques. The rate of deep infection and osteomyelitis with these techniques remains up to 20% in some studies.45 Surgeons who prefer this technique should consider burying the K-wires during the consolidation phase, as infection and nonunion rates are much lower with buried techniques are utilized.46
Nonunion
Reported nonunion rates for DIP arthrodesis range from 0–20%. Several studies have reported low rates of nonunion with screw techniques44,45,47–49 as well as traditional techniques,46 however a clear relationship between fixation technique and the rate of nonunion has not been shown for DIP arthrodesis.45
Kirschner wire and interosseous wire techniques produce significantly less compression across the fusion site than compression screw techniques and compression itself has been shown to achieve more rapid fusion. In a biomechanical cadaver study comparing fixation techniques, the use of a single buried axial compression screw (Herbert screw) was shown to be superior in terms of fixation strength when compared to a combined K-wire/tension band technique.42 Despite this biomechanical study, retrospective clinical studies have noted similar nonunion rates when comparing compression screws, interosseous wiring, and crossed K-wires techniques; it is thought that nonunion rates most closely correlate with the amount cortical bone and condition of the bone stock at the time of operative procedure.45
DIP arthroplasty
Distal interphalangeal joint arthroplasty is an uncommon procedure with few indications. The obvious advantage is maintained range of motion of the DIP, which may be beneficial in selected cases, e.g., in professional musicians.30 There are few published series and these all report on the use of silicone interposition implants.27–29 The largest series reported 38 DIP arthroplasties of which 10% were removed by 10 years. The average range of DIP motion was 33° with an average extension lag of 12°.28
Mucous cyst
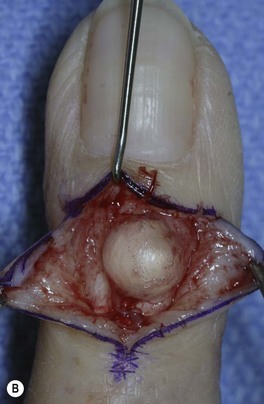
Dorsal synovial cysts are commonly associated with DIP arthritis and develop in response to irritation of the joint by marginal osteophytes (Fig. 20.3). Cysts slowly expand damaging the germinal matrix resulting in nail deformity and nail grooving. Progressive thinning of the overlying skin and recurrent inflammation are also common. Infection can be troublesome, as communication with the joint risks the development of septic arthritis and recurrently symptomatic cysts should be removed surgically.
Needle aspiration of the mucous cyst with subsequent corticosteroid injection of the DIP joint is appealing because it can be performed in the office setting with minimal morbidity.50–52 Typically, the cyst is too small for effective needle aspiration and instead, multiple punctures are made with a 25-gauge needle and manual expression of the cyst fluid is performed. The joint may then be injected with a lidocaine and corticosteroid solution and wrapped snuggly for several days. This technique has been associated with a 60% resolution of the cyst.50 However, one must observe careful sterile technique to minimize chance of infection into the joint space.
If the cyst recurs surgical excision can be performed. Our preferred surgical technique consists of marking the margins of the cyst under loupe magnification followed by excision of the cyst and overlying attenuated skin. The underlying inflamed synovium should be debrided and associated dorsal and marginal osteophytes are removed. Specific attention should be paid to preservation of the extensor insertion, which can be challenging as it may be significantly attenuated. Skin closure may require the use of local skin flaps in rare circumstances.50,53–55
Fritz et al. reported the results of surgical excision of 86 mucous cysts. Nail deformities were present in 29% of patients preoperatively.56 Postoperative loss of extension of 5–20° at the IP or DIP joints was seen in 17% of patients. One patient developed a superficial infection and two developed a DIP pyarthrosis, eventually requiring DIP arthrodesis. A total of 7% of patients without preoperative nail deformity developed one postoperatively; however 60% of preoperative nail deformities were improved by the procedure. Recurrence was seen in 3% of cases and other complications included persistent swelling, pain, numbness, stiffness, and radial or ulnar deviation at the DIP joint.
Proximal interphalangeal (PIP) joint arthritis
Within the hand, the PIP joint is the third most frequently affected by OA. The joint is more commonly affected in females.25 The PIP joint has been described as the “functional locus of the finger” as it produces 85% of intrinsic digital flexion and contributes 20% to the overall arc of finger motion.57 Despite this, a full range of motion is not absolutely necessary for good hand function, and a 45–90° flexion arc allows for most activities.
As in DIP arthritis, PIP joints affected by OA are often enlarged and painful. Patients will complain of their inability to wear or remove rings from the affected fingers. Bouchard’s nodes are pathognomonic for PIP OA and are bony protuberances at the joint, analogous to Heberden’s nodes at the DIP joint. Range of motion at the PIP joint tends to be preserved until late in the disease process. Radiographic findings include joint space narrowing, subchondral sclerosis and osteophytes (Fig. 20.2).
Management
Patients with occasional or minor symptoms are medicated with oral or topical non-steroidal anti-inflammatory drugs. Resting the joint by splinting in extension can be used to decrease pain in acute exacerbations. Intra-articular steroid injections can also be used to decrease pain but repeated injections are impractical for the long-term management of severely symptomatic patients. When nonoperative management strategies are insufficient to control the patient’s symptoms, PIP joint OA is usually treated surgically, with either arthrodesis or implant arthroplasty. Vascularized joint transfer is technically feasible but uncommonly practiced due to significant donor site morbidity and inferior outcomes when compared with implant arthroplasty.58–61
The decision for PIP arthrodesis or arthroplasty is dictated by the patient’s functional needs, desires to preserve PIP motion, and the requirements for lateral stability at the joint. The ulnar three digits are important for generating grip strength. Maximal grip strength requires almost full PIP flexion to avoid the quadriga effect. The index finger’s relatively independent FDP function does not impose a significant quadriga effect on the remaining digits during grasp. In comparison, the index finger is used to generate oppositional and adduction pinch strength, which requires PIP lateral stability but little PIP motion if metacarpophalangeal joint motion is preserved. Therefore, the decision for arthrodesis versus arthroplasty may be different for each digit involved. For example, arthrodesis of the index finger PIP joint provides finger stability for pinch and is well tolerated; however for the ring and small finger PIP joints, implant arthroplasty may be preferred as PIP joint motion in these digits is important for grip function and strength. If fusion is to be performed on the PIP joints of the ulnar three digits care must be taken in placing the joints in enough flexion to preserve grip strength. A significant decrease in grip strength occurs when the PIP joint of the small finger is fused in less than 45° of flexion, and when the PIP joint of the middle and ring fingers are fused in less than 60° of flexion.62
PIP arthrodesis
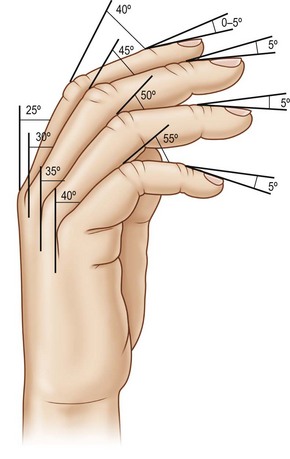
PIP arthrodesis can be accomplished using the same techniques as described for DIP arthrodesis. The optimal position of PIP fusion varies according to the digit, with some authors recommending 40° of flexion for index, 45° for the long, 50 for the ring and 55° for the small finger (Fig. 20.5);63 however, this can be tailored according to the patient’s occupation and recreational activities. Preoperative splints simulating fusion at various angles can be used to allow the patient to make a more informed decision.
Techniques
Compression screw
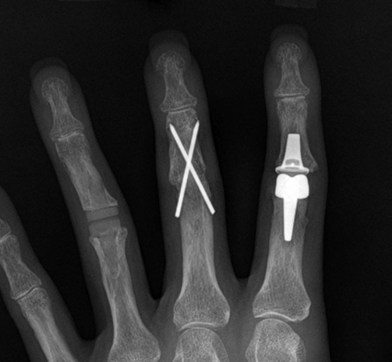
Arthrodesis of the PIP joint can be accomplished by antegrade insertion of a headless compression screw across the prepared joint (Fig. 20.6A). A K-wire is used to start a hole in the dorsal cortex of the proximal phalanx and oriented obliquely in a sagittal plane. The angle of insertion should correspond to the desired angle of fusion and the wire is inserted antegrade across the joint, into the middle phalanx. The position of the entry hole in the proximal phalanx should be at least 6 or 7 mm proximal to the joint to prevent fragmentation of the dorsal cortex, which can be a complication of this technique. The K-wire is used as a guide wire for the cannulated drill sequence required for the particular screw being used (as described above for DIP arthrodesis). Fluoroscopy is used to check alignment, and to choose an appropriately sized screw that is then inserted in an antegrade fashion, with the assistant holding manual compression of the bone surfaces. The thread should reach the cortical isthmus of the middle phalanx to achieve endosteal purchase. The dorsal soft tissues are closed and a bulky splint is applied. A thermoplastic splint can replace this after 7 days, which should be worn for approximately 3 weeks. The major advantage of this method of fixation is the substantial stability of the construct, which allows early mobilization, and less likelihood of hand stiffness (Fig. 20.7A).
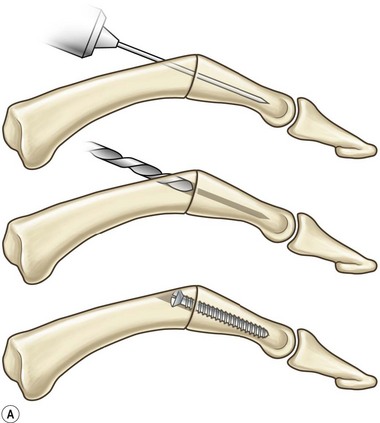
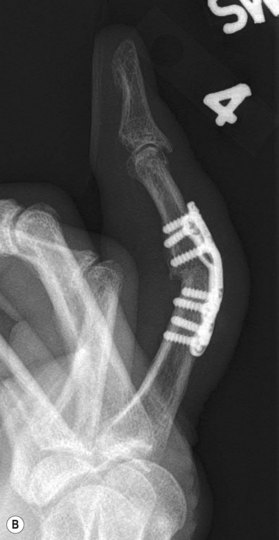
Fig. 20.6 Example of a PIP fusion with (A) headless compression screw and (B) dorsal plating technique.
Plating
For severely osteoporotic bone, or for salvage of failed fusion attempts using other methods, PIP arthrodesis may be obtained using mini-plates (1.7 mm up to 2.7 mm). Newer plate designs allow for compression or locking screw placement which can significantly improve fixation in severely osteoporotic bone. Plates are applied using standard AO technique. Pre-contouring of the plates is required to achieve the appropriate angle (Fig. 20.6B).
PIP arthroplasty
Arthroplasty will allow for some preservation of joint motion. Arthroplasty techniques consist of soft tissue interposition, silicone spacers and a variety of constrained and unconstrained implants. Soft tissue arthroplasties are mentioned for historical purposes but have been largely replaced by implants, silicone or other. Techniques for soft tissue arthroplasty have included resection of the head of the proximal phalanx,64 volar plate interposition,65 and perichondrial transplant arthroplasty.66–68
Silicone interposition arthroplasty
Silicone interposition implants consist of one-piece flexible, stemmed, hinged elastomer spacers that can be used in all digits after resection arthroplasty of the PIP joint. Swanson and Niebauer first devised these implants in the 1960s (Fig. 20.7). The implant consists of a single silicone unit with a tapered proximal and distal stem with a central interposed flexion region, which is dorsally offset (Fig. 20.8A). The implant does not function as a true joint but rather a spacer that is stabilized by the fibrous capsule, which develops around the implant. The implant glides or “pistons” within the medullary canals during movement and this was originally believed to disperse forces over a broader surface area potentiating the life of the implant. Newer implant designs have maintained the same general concept but have incorporated a 30° pre-flexed hinge between the proximal and distal stems to facilitate finger flexion.
Technique
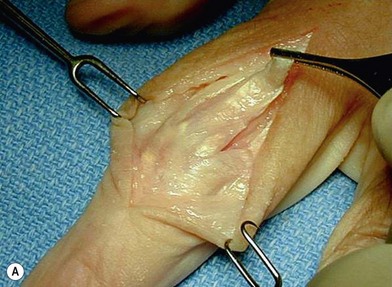
The PIP joint can be exposed by one of three surgical approaches – dorsally, laterally or from the volar surface. We prefer a longitudinal dorsal approach in most cases, as it affords superior exposure to the joint and allows easier insertion of the implants. A 2–3 cm straight or curved dorsal incision is made over the PIP joint and continued through the center of the extensor tendon from mid-proximal phalanx until just distal to the insertion of the central slip on the middle phalanx. The two parallel halves of the central slip are then mobilized off the underlying middle phalanx. This produces radial and ulnar tendinous bands, each containing half of the central slip and a lateral band, which are allowed to sublux laterally when the middle phalanx is flexed, exposing the PIP joint. The joint is flexed to 90°, and osteophytes are removed as necessary. An alternative exposure of the PIP joint involves the use of Chamay technique, where a distally based flap of the extensor mechanism is elevated from proximal to distal (Fig. 20.8). The flap is then repaired at the completion of the surgical procedure.69
Surface replacement arthroplasty with nonconstrained implants
The first PIP joint replacement prosthesis was described in 1959 by Brannon and Klein.70 Numerous implant designs have since been produced and many have been abandoned due to implant failure, instability or high complication rates.71–74 At present, the two designs available in the United States are made of metal or PyroCarbon.
Linscheid and Dobyns introduced the first nonconstrained PIP prosthesis in 1979 and this design is still available as the SR-PIP implant (Small Bone Innovations, New York – previously Avanta, San Diego).75 The proximal component is made of a chromium-cobalt alloy with a polished articular surface, and the distal component has an ultra-high molecular weight polyethylene (UHMWPe) articular surface fixed to a titanium base and stem. The articulating surfaces is a tongue in groove configuration, similar to the PIP joints anatomic design. For this reason, the system is described by the manufacturer as “semiconstrained.” This congruency adds to the lateral stability of the joint. The titanium stems are textured and are designed to “press-fit.” However, noncemented implants have been shown to have a higher incidence of subsidence and loosening.76
Technique for nonconstrained or semiconstrained PIP arthroplasty
Surface replacement arthroplasty of the PIP joint can be performed through either a dorsal, lateral, or volar approach (Fig. 20.9). Our preference is for the dorsal approach as it provides excellent exposure of the joint and facilitates implant placement. A 2–3 cm straight or curved dorsal incision is made over the PIP joint and continued through the center of the extensor tendon from mid-proximal phalanx until just distal to the insertion of the central slip on the middle phalanx. The 2 parallel halves of the central slip are then mobilized off the underlying middle phalanx. This produces radial and ulnar tendinous bands, each containing half of the central slip and a lateral band, which are allowed to sublux laterally when the middle phalanx is flexed, exposing the PIP joint. The joint is flexed to 90°, and osteophytes are removed as necessary.
Postoperative therapy for PIP arthroplasty
Patients must adhere to strict hand therapy follow-up for approximately 10 weeks following surgery and this should be discussed preoperatively, as poor therapy compliance will lead to poor outcome (Fig. 20.10).77 Hyperextension of the PIP joint must be avoided at all costs, and we therefore do not pursue complete joint extension, preferring to accept a 5–10° flexion deformity, as hyperextension will lead to joint dislocation.
Outcomes and complications of PIP arthroplasty
Successful implant arthroplasty of the PIP joint affords obvious benefits over arthrodesis; however, despite multiple advancements in implant design, uniformly predictable results have been difficult to achieve.78 The major complications from PIP arthroplasty continue to be implant failure, instability and loss of motion. Pellegrini and Burton compared arthrodesis and silicone and metal arthroplasty in 43 patients and noted better pinch strength in the arthrodesis group especially in the index finger. Few revisions were necessary in the silicone arthroplasty group, however seven patients underwent revision of the metal prosthesis in order to improve lateral stability. All of these failed and required revision, and the authors concluded that arthrodesis remains the treatment of choice for the index finger, and silicone arthroplasty for the ulnar digits.79
Silicone implants have been used for PIP replacement for over 40 years with several large series’ reported in the literature. Pain relief tends to be good and the degree of functional improvement and patient satisfaction is high; however, very few series’ report improvement in range of motion. The reported incidence of implant fracture with silicone arthroplasty is variable, ranging from 5–44%, and rotational malalignment and lateral deviation may occur with longer follow-up.80–82 The first large series of silicone interposition arthroplasty was reported by Swanson in 1972.83 He reviewed 148 implants and there were no major complications. The series was later expanded to report 424 PIP joint arthroplasties. In this series, complete pain relief was achieved in 98% of patients, with an average increase in the arc of PIP motion of 10°.84 Complications included bone resorption in 1.2% of cases and implant fracture in 5%. More recently, Takigawa et al. reviewed 70 implants with an average follow-up of 6.5 years and reported pain relief in 70%, improvement in extension from 32° to 18°, but no change in total active motion of the PIP joint.85 Complications included fractures in 15% of the implants, and nine joints required revision. Patients rated their outcome as good in 25 joints, fair in 27, and poor in 18.
Lin et al. reviewed their experience of 69 PIP silicone arthroplasties done through a volar approach.81 There was an improvement in the extension deficit from 17° to 8° but no improvement in overall arc of motion. Complete resolution of preoperative pain was noted in 67 of 69 joints and complications were observed in 12 joints, including five implant fractures and three cases of implant malrotation. More recent studies by Namdari and Weiss reported 4-year outcomes of 16 joint replacements using the pre-flexed NeuFlex silicone implant.86 After an average 4-year follow-up, the mean range of motion was 61°, with 0° of extension lag. Pain relief was excellent or good in 84% and overall patient satisfaction was rated at 90%. Pettersson et al. compared this new implant with a traditional silicone implant design, and found better patient satisfaction but otherwise similar results.87 Silicone interposition PIP arthroplasty remains popular and is often used as a benchmark comparison for newer surface replacement implants such as metal or PyroCarbon systems.
The outcomes of metal surface replacement arthroplasty was reported by Linscheid et al., where they described the outcomes of 66 cemented CoCr-UHMWPE surface replacement prostheses with an average 4.5-year follow-up.71 In this study, the average arc of motion improved from 35 to 47°, and total pain relief was achieved in 85%. There were a significant number of complications, including five swan-neck deformities; five unstable joints; coronal deviation in four joints, and 12 joints required reoperation.
Jennings and Livingstone reviewed 43 metal surface replacement PIP arthroplasties with an average follow-up of 37 months, and found no change in arc of motion and a 25% revision rate.88 Revision arthroplasties were generally performed for loosening of the components. They found a significantly higher loosening rate for uncemented components (39%) when compared with cemented components (4%). Johnstone et al. reported similar findings in their study.76 Johnstone and colleagues noted a higher incidence of subsidence and component loosening in newer generation implants which were not cemented.76 Luther et al. recently reported an average 27-month follow-up on 24 SR™ PIP arthroplasty.89 They found that 14 joints required reoperation (58%), including four that required removal. Despite this, 70% were satisfied with their final functional results.89
The results of pyrolytic carbon arthroplasty for proximal interphalangeal joint replacement have been the subject of several recent studies.90–94 Bravo et al. published the largest and most comprehensive study to date.91 A total of 50 PIP PyroCarbon joint replacements were performed in 35 patients. The minimum follow-up was 27 months. The average age at the time of surgery was 53 years. Joints replaced included the index,15 middle,18 ring,10 and small.7 The mean preoperative range of motion was 40°, mean pinch and grip measurements were 3 and 19 kg, respectively, and the mean preoperative pain score was 6 by visual analog scale (0–10). Postoperatively, mean range of motion improved to 47°, mean pinch and grip measurements were 4 and 24 kg, respectively. Despite these modest improvements, the mean postoperative pain score was 1 and overall patient satisfaction was high (80%). Fourteen joints required additional procedures and the revision arthroplasty rate was 8%. There were no cases of implant infection. Notably, radiographic evidence of subsidence was noted in 20 joints although there was no apparent loosening.
Tuttle et al. also reported a retrospective series of 18 joint replacements in eight patients.93 The average active range of motion was unchanged, however there were no cases of lateral instability. Complete resolution of preoperative pain was apparent in only eight cases. Complications included two dislocations evident at the first office visit; five contractures (with a motion arc of <35°), and eight squeaky joints. Wijk et al. reported 2-year follow-up on 53 PIP PyroCarbon arthroplasty procedures.94 Range of motion and grip strength were unchanged postoperatively, however pain relief and occupational performance were improved and patient satisfaction was high. Revision surgery was required in seven patients, including two who required subsequent arthrodesis.
Chung et al. evaluated 14 patients (21 joints) prospectively. The mean arc of motion at 12 months was 38°, grip and pinch strength both improved postoperatively. Patient-rated outcomes were studied by questionnaire, showing a significant improvement in pain, satisfaction and esthetics outcome.58
Herren et al. performed 17 PyroCarbon PIP joint arthroplasty procedures for osteoarthritis, which were followed prospectively for a mean of 20.5 months.95 All patients noted significant pain relief with a reduction in mean preoperative pain score of 7.6 on a 10-point VAS, to 1.3 at final follow-up. One patient required a revision procedure because of implant migration. Nunley et al. performed five PyroCarbon PIP arthroplasties on younger patients with posttraumatic arthritis.92 These were followed prospectively for 1 year and were found to have no statistically significant improvement in pain or motion arc, however grip strength increased significantly postoperatively.
Branam et al. have published the only study comparing the outcomes of pyrolytic carbon implants with silicone implants for PIP arthroplasty.90 This retrospective study compared outcomes of 19 PyroCarbon implants with 23 silicone implants. Although differences in final motion arc and pain relief were statistically insignificant, coronal plane deformity was more prevalent and more pronounced in patients who had silicone arthroplasty. Overall it is apparent that both joints provide benefits with regard to pain relief and preservation of some motion. The superiority of one type of implant over another has yet to be determined.
OA of the metacarpophalangeal (MCP) joint
Anatomy and biomechanics
The metacarpophalangeal (MCP) joint is a multiaxial condyloid joint that allows for flexion, extension, abduction, adduction, and a limited degree of circumduction. Circumduction or rotation is not a motion under active control, but is allowed for passively by the intrinsic joint mechanics. The joint contributes to 77% of the total arc of finger flexion.96 The metacarpal head has been described as having a unique “cam” shape. However, this description is not true to its mechanical reality. A cam is mechanical link that translates rotational motion into linear motion or vice versa, typically with a rotating eccentric wheel. However, the head of the metacarpal is round and therefore, the analogy is not valid. The circular metacarpal head is offset volar to the access of the metacarpal shaft, which does result in differential tension of the collateral ligaments in flexion and extension. The unique three-dimensional shape of the head, being wider on its volar aspect, adds to the collateral ligament tension in joint flexion and limited lateral motion in this position. On the other hand, when the MCP joint is extended the collateral ligaments are lax and there is maximal abduction and rotation of the joint in this position.
The MCP joint capsule is composed of areolar tissue dorsally that is loosely reinforced by the extensor tendon and the sagittal band.97,98 Laterally, the collateral ligaments support the joint, while the volar plate provides volar support. The intrinsic muscles provide only modest, secondary capsular support. The ulnar and radial proper collateral ligaments are fan-shaped, thick and wide, arising from the dorsal aspect of the metacarpal head and inserting into the volar aspect of the proximal phalanx. The accessory collateral ligaments are located volar to the origin of the proper collateral ligaments on the metacarpal head and interdigitate with the proper collateral ligaments distally. The collateral ligaments play a major role in stabilizing the MCP joint in all directions of joint displacement. The accessory collateral ligaments contribute primarily to abduction-adduction and rotational stability, while the volar plate prevents dorsal dislocation of the joint when fully extended. The dorsal capsule provides a very modest contribution to stability when the joint is in distraction and at the extremes of supination and pronation.97,98
Osteoarthritis (OA) affects the MCP joint less commonly than other hand joints and is usually secondary to injury or trauma, e.g., intra-articular fracture, septic arthritis, chronic repetitive trauma in setting of heavy labor occupations, and occasionally in metabolic disorders such as hematochromatosisi.99–101 MCP OA usually affects the index and middle fingers.99,100 Conservative treatment with splinting, hand therapy, non-steroidal anti-inflammatory drugs, and steroid injections is the first-line treatment of MCP OA. Surgery is indicated for stiffness and/or pain that are refractory to nonoperative treatments.
Resection and resurfacing arthroplasty
Resurfacing arthroplasty involves the resection of diseased metacarpal cartilage and bone, followed by the placement of soft tissue into the joint space to prevent direct bone contact. This procedure was used historically to treat MCP OA but now is reserved as a salvage procedure for cases of failed implant arthroplasty. Tupper102 described the use of the volar plate for interposition arthroplasty. The volar plate is detached proximally from the metacarpal head before the diseased cartilage and bone of the metacarpal head is resected. This distally based volar plate is then attached to the dorsal edge of the osteotomy by means of sutures preplaced through drill holes in the metacarpal. Before the joint capsule is closed, the collateral ligaments are reattached using horizontal mattress sutures preplaced through drill holes on the sides of the distal metacarpal.103,104 Other resection/interposition arthroplasty techniques using tendon and interosseous muscles have been described by Vainio105 and Riordan and Fowler,106 though not specifically for OA.
Costal perichondrium may also be used for joint resurfacing. After harvesting the perichondrium and cutting it to the size and shape of the joint surface, the perichondrial graft is secured by means of a horizontal mattress suture. There are limited and variable reports on the outcomes of using rib perichondrium for resurfacing arthroplasty.67,107 A study by Seradge67 noted poor outcomes in patients over 40 years of age and uniform failure in cases of reconstruction after septic arthritis. He concluded that such patients constitute a contraindication for the procedure. If perichondrium is to be used it should be oriented with the deep surface facing the joint as this is the chondrogenic surface. Similarly if the retinaculum or volar plate are used for resurfacing, the synovial (deep) surface should be oriented facing the joint.107
Implant arthroplasty
Hinged prostheses
Metal-hinged prostheses are rarely used today. The 1953, Brannon and Klein prosthesis was the first prosthesis to be used for MCP arthroplasty.70 This simple, uniaxial metal prosthesis resulted in bone resorption with subsequent finger shortening and loss of range of motion. In the 1970s and 1980s, the hinge implants evolved into multi-axial designs made of metal, polymers, or ceramics. The two-component ball-and-socket Griffith-Nicolle prosthesis108 and ceramic prostheses109,110 were strong enough to prevent fracture, but postoperative range of motion was too limited for adequate joint function.108
Silicone constrained prostheses
The Swanson prosthesis was the first silicone MCP joint prosthesis and has been in use since the 1960s.111 This prosthesis has been widely used and studied, although primarily for rheumatoid arthritis (RA). The silicone elastomer is cross-linked which creates a rubber-like form to the implant. The implant acts as a dynamic spacer and not a true joint. The prosthesis is held in position by a process of fibrous encapsulation, instead of osteointegration.111 The prosthesis does not flex at the central hinge section until the joint has been flexed beyond 45°. Instead, in early flexion, the implant bends at the interface between the stem and the hinge.112 This results in a high incidence of stem fracture. Beckenbaugh reported a fracture rate of 26% at 2.5 years when using the original Swanson MCP prosthesis. After this study, the silicone elastomer used in the prosthesis was modified to increase its tear propagation strength and its fatigue-crack growth resistance, and a newer, “High Performance implant” was released.113 Kirschenbaum et al.114 reported a 10% fracture rate within 8.5 years of insertion of the modified Swanson implant. Fracture rates of around 5–7% have been reported at 2–5-year follow-up.115,116 Interestingly, many of these fractures do not require treatment as they go unnoticed by the patients.114 Other complications with the implant have included implant deformation and silicone synovitis.114,117,118
The clinical experience with the Swanson implant in patients with RA constitutes the main body of the literature on this implant. However, Rettig et al.119 reported their experience with silicone MCP arthroplasty in the setting of OA. While the experience in RA can shed some light on the management of patients with OA, the outcome of silicone arthroplasty in this population is likely to be different than patients with OA. They reported 100% patient-reported overall improvement with a mean MCP flexion of 59°. This small study consisted of 12 patients and 14 MCP joints. In contrast to the literature on Swanson implant failures in the setting of MCP RA, this study, albeit small, demonstrated no Swanson implant fractures in patients with OA.
The limitations of the Swanson implant in patients with RA have motivated the development of new silicone implant designs like Sutter (Avanta) and the NeuFlex (DePuy, Warsaw, IN) implants. Unfortunately, computer modeling has shown higher stresses in the Avanta design when compared to the Swanson implant,120 and this has been confirmed clinically with observations of at least double the fracture rate in patients with RA.121 Studies are more promising for the NeuFlex implant, an anatomically neutral silicone implant with a block-hinge design and a preformed 30° resting angle that approximates the relaxed MCP position. The logic for this design is that with the finger in repose, there is no stress on the implant, contrary to the Swanson design. In vitro studies also suggest that the NeuFlex implant has greater longevity than the Swanson implant before fracture.122 Weiss et al.123 compared MCP joint mechanism after NeuFlex, Swanson and Sutter arthroplasty and found that the mechanics of the NeuFlex implant most closely matched that of an intact MCP joint. Only one study86 specifically examines the in vivo use of NeuFlex implants in OA of the MCP joint. At 4-year follow-up, NeuFlex implants were associated with a mean flexion arc of 65°, a mean extension lag of 3°, no implant fractures, and high patient satisfaction.86 Despite the limitations of the Swanson MCP prosthesis, it remains the current “gold standard” to which other implants are compared. Not only is it effective in relieving pain and improving motion arc, but it is also relatively cheap and easy to place and remove (Fig. 20.11).124–126
Surface replacement prostheses
Silicone implants are inadequate in patients who place a higher demand on the MCP joint due to employment or lifestyle. Surface-replacement prostheses are more appropriate in such patients unless the soft tissue around the MCP joint is inadequate to offer implant stability.127–130 Surface-replacement prosthetics attempt to recreate normal joint anatomy. They vary in their design: a single-component silicone implant with a Dacron-coated stem;127 a nonjoined, two-component pyrolite carbon implant;128 a four-component polyethylene-titanium implant;129 or a three-component titanium plate-silastic hinge implant.130 Studies on surface replacement prostheses are small, consisting mainly of RA patients, with short postoperative follow-up. With the exception of a 20% fracture rate with the Hagert prosthesis, the surface replacement prostheses are associated with a high patient satisfaction, resolution of pain, improved cosmetic appearance, average range of motion of 47–60°, and a low fracture rate.127–130 The main problem with these implants is instability and dislocation.128
Kung et al. developed a stable surface replacement arthroplasty implant based on cadaveric evaluation of normal MCP joint articular geometry.131 The implant is a non-constrained, two-component implant with a hemi-spherical cobalt chrome alloy metacarpal component with and an ultra high molecular weight polyethylene phalangeal component. The head has lateral volar contours that increase lateral stability during flexion. Also, its arc of rotation is greater than that of an anatomical joint to decrease the risk of palmar subluxation. The stem of each component may be “press-fit” or secured into the intermedullary canal with bone cement. When mechanically tested at different angles, axial loads, and directions of motion, this semiconstrained MCP joint implant demonstrates more intrinsic stability than cadaveric MCP joints.131
PyroCarbon arthroplasty
Pyrolytic carbon, formed by the pyrolysis of a hydrocarbon gas, has an elastic modulus similar to cortical bone.132 This makes the material ideal for implant-bone stress transfer, potentially reducing implant wear and subsidence.132 The implant is stabilized by appositional bone growth and does not require cement fixation.133
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


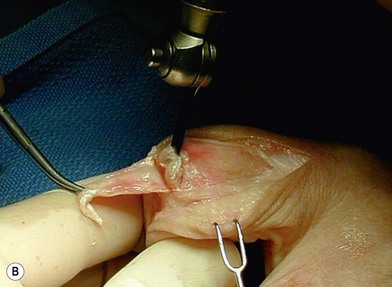
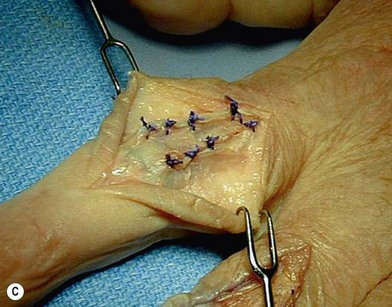
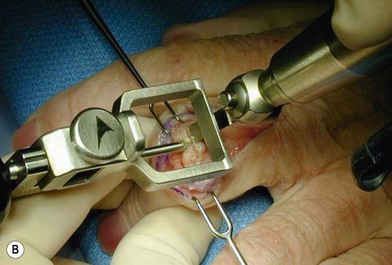
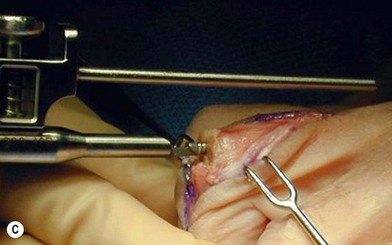
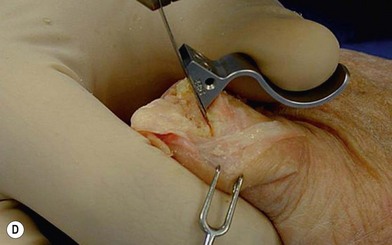
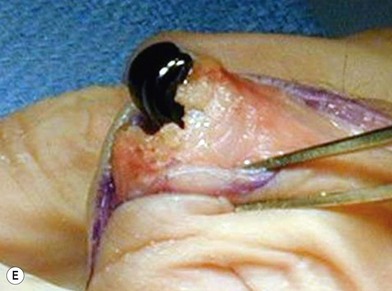
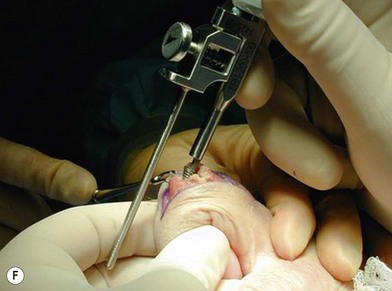
 of the proximal phalanx head, and fluoroscopy is used to confirm a central position in the medullary canal. A starter awl is then used to enlarge the intramedullary canal. A cutting guide is then used to create a vertical osteotomy approximately 0.5–1 mm distal to the insertion of the collateral ligaments on the head of the proximal phalanx removing the articular cartilage. Next, the proximal phalanx is broached, until the medullary canal is filled with the largest broach possible. The lateral fluoroscopic view will be the first to show the size limitations of the intramedullary canal. Once the canal has been enlarged, a matched oblique cutting guide is used to create a back cut in the proximal phalanx, congruent with the sized implant shape. A proximal trial implant can then be inserted and checked radiographically for size and alignment.
of the proximal phalanx head, and fluoroscopy is used to confirm a central position in the medullary canal. A starter awl is then used to enlarge the intramedullary canal. A cutting guide is then used to create a vertical osteotomy approximately 0.5–1 mm distal to the insertion of the collateral ligaments on the head of the proximal phalanx removing the articular cartilage. Next, the proximal phalanx is broached, until the medullary canal is filled with the largest broach possible. The lateral fluoroscopic view will be the first to show the size limitations of the intramedullary canal. Once the canal has been enlarged, a matched oblique cutting guide is used to create a back cut in the proximal phalanx, congruent with the sized implant shape. A proximal trial implant can then be inserted and checked radiographically for size and alignment.