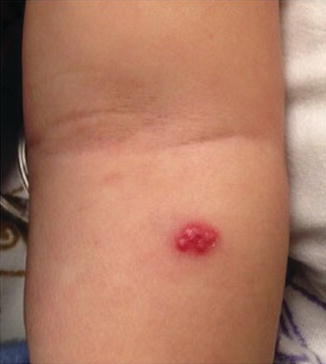
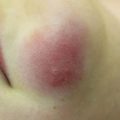
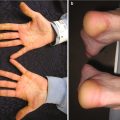
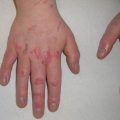
Fig. 10.1
Widespread cutaneous methicillin-sensitive Staphylococcus aureus infection in a renal transplant recipient (image courtesy of Carrie C. Coughlin, MD)
Cellulitis is a superficial infection of the dermis and/or subcutaneous tissue typically from a bacterial infection. Necrotizing fasciitis and muscle infection (myonecrosis ) are deeper infections that can be especially severe in immunosuppressed patients. Although S. aureus and group A Streptococcus (GAS) are more common causes of these deep bacterial infections, necrotizing fasciitis and myonecrosis in immunosuppressed patients are often polymicrobial, including gastrointestinal flora and anaerobic causes [13]. Due to the lack of a typical immune response in immunosuppressed patients, the clinical findings of erythema and induration may be missing or subtle in these deeper infections, thus complicating diagnosis [13]. Severe neutropenia after induction chemotherapy is a significant risk factor for these bacterial infections [14]. Therapy is similar to treating immunocompetent hosts except that the initial empiric antibiotic therapy should be broad and include gram-positive, gram-negative, and anaerobic bacterial coverage.
Coagulase-negative staphylococci such as Staphylococcus epidermidis are part of the normal bacterial microbiome of the skin and not typically pathogens. In fact, S. epidermidis plays an important role in cutaneous immunity by secreting antimicrobial peptides, in addition to activating Toll-like receptor 2, helping augment the innate immune system [15]. S. epidermidis is an important cause of bloodstream infections in very low-birth-weight neonates and immunosuppressed patients, though it does not usually cause primary cutaneous infection [16].
Aerobic Gram-Negative Bacteria
Ecthyma is a necrotic infection of the skin and soft tissue due to vascular compromise in the infected area. The term “ecthyma gangrenosum ” is typically reserved for ecthyma caused by the oxidase-positive, gram-negative rod Pseudomonas aeruginosa . In immunosuppressed patients, the clinician should have a broad differential diagnosis for a purple eschar including infection by bacteria such as Escherichia coli , Aeromonas , GAS and Serratia , as well as invasive molds such as Aspergillus and Zygomycetes [17]. Lesions of ecthyma gangrenosum can occur due to direct inoculation of bacteria into the skin or from hematogenous spread. P. aeruginosa often colonizes stool; therefore, especially in diapered infants, primary lesions may be in the perineum [18, 19]. The primary lesion is a purple macule or patch that upon palpation often has underlying induration. The initial lesion rapidly progresses to a hemorrhagic bulla or ulcer with an indurated margin. If the cutaneous infection was caused by hematogenous spread to the skin, the child will typically have fever. However, if the lesion was inoculated into the skin from an outside source, the patient may initially be afebrile. Pseudomonas rarely infects normal hosts, so the presence of ecthyma gangrenosum should alert a clinician to evaluate for immunodeficiency such as neutropenia or a newly presenting hematopoietic malignancy [20]. A biopsy with frozen section (if possible) as well as culture can help rapidly determine if the cause is bacterial (gram negative or positive) or fungal, helping to guide empiric therapy. Choosing empiric coverage for Pseudomonas is challenging due to geographically different resistance patterns [21]. Some experts advocate for initial empiric therapy with two antipseudomonal antibiotics that have different mechanisms to increase the likelihood of successful treatment for this life-threatening infection until susceptibility testing can help narrow the coverage [18, 21, 22].
Anaerobic Infections
Corynebacterium and other diphtheroids are common colonizers of the skin that are difficult to differentiate from contaminant in cultures. Anaerobic bacteria can cause coinfection of surgical sites [23]. They can also be present as a coinfection in abscesses and deeper infections such as myonecrosis and necrotizing fasciitis [13]. Culturing anaerobes is more challenging because the collection requires anaerobic conditions and the laboratory must be aware to consider any growth, and not assume that anaerobes are contaminants.
Viral Infections
Key Points
Herpesviruses establish latency following primary infection, allowing for recurrence in select hosts.
HSV may present at multiple or atypical sites, or manifest as hyperkeratotic or verrucous lesions.
VZV infection may result in chronic or disseminated disease.
EBV may cause lymphoproliferative disease with rare cutaneous manifestations.
Kaposi sarcoma can be confused with benign vascular tumors, and may have additional systemic signs.
HPV and molluscum contagiosum lesions can be extensive, atypical appearing, and refractory to treatment.
Herpesviridae
The herpesvirus family, consisting of HSV1/2, VZV/HHV-3, EBV/HHV-4, CMV/HHV-5, HHV-6, HHV-7, and KSHV/HHV-8, are relatively ubiquitous, double-stranded DNA viruses. They are further divided into subfamilies (alpha-, beta-, and gamma-herpesviruses) based primarily on the length of their reproductive cycles and the cell types in which they establish latency. While herpesvirus infection in the immunocompetent host is rarely severe, these viruses can cause atypical or disseminated infections in immunocompromised patients, leading to significant morbidity and mortality.
Herpes Simplex Virus (HSV)
HSV-1 and HSV-2 are alpha herpesviruses. Primary infection and replication occur within mucocutaneous sites, followed by retrograde axonal flow extending to the dorsal root ganglion. The virus can remain latent for long periods within the dorsal root ganglia, thus avoiding detection by the host immune system. HSV-1 is the dominant serotype among young children [24]. It is typically acquired between the ages of 2 and 10 through contact with contaminated oral secretions [24]. In contrast, primary infection with HSV-2 more commonly occurs after puberty through anogenital contact, and remains the leading serotype associated with genital herpes worldwide [24]. Many factors can trigger reactivation, including immunosuppression. Viral culture , Tzanck smear , and direct fluorescence antibodies (DFA) have classically been used as initial diagnostic tests; however polymerase chain reaction (PCR) is now the gold standard for diagnosis due to its high sensitivity and specificity, in addition to its rapid turnaround time [25].
HSV classically presents as tender, grouped, erythematous vesicles that can become infiltrated with inflammatory cells leading to a pustular appearance. Tingling or burning may precede lesions in both primary and recurrent infections. In immunosuppressed children, the immune system may have difficulty clearing the virus from the skin. Reactivation occurs more frequently when there is impaired cell-mediated immunity, and exhibits both more prolonged symptom duration and viral shedding [24].
In immunosuppressed patients, lesions are more likely to affect multiple sites. Large, hyperkeratotic, verrucous, ulcerated, and exophytic plaques have been reported in adults, but can present in children as well [26]. Lesion location also differs in immunosuppressed hosts; there is more frequent intraoral involvement, most notably affecting the gingiva, palate, or buccal mucosa [27]. Deep, linear fissuring (“knife-cut sign”) can occur in intertriginous areas, as well as on the tongue dorsum (herpetic geometric glossitis) [28]. HSV can disseminate to the lungs, liver, GI tract, and central nervous system, although this is rare. Lesions may be preceded by fever, lymphadenopathy, and malaise. In primary HSV gingivostomatitis of an immunosuppressed host, there can be severe involvement of the oral cavity. Pharyngitis is more common in older children and adolescents.
Intravenous (IV) acyclovir is the mainstay of treatment for severe and disseminated infections, as well as for patients with systemic complications [29]. Unlike in immunocompetent hosts, oral and IV antivirals should be continued until lesions are completely healed. Acyclovir-resistant strains of HSV may occur, particularly among immunosuppressed patients. Degree of immunosuppression and prolonged or erratic acyclovir use are risk factors in developing resistant strains [30]. Persistent lesions without appreciable decrease in size after more than 1 week of treatment, development of atypical lesions, or appearance of new satellite lesions after 3–4 days of therapy suggest resistance, and treatment with foscarnet or systemic cidofovir may be considered [29].
Varicella Zoster Virus (VZV)
VZV is the third member of the alpha-herpesvirus subfamily. It is transmissible through contact with respiratory secretions or fluid from skin lesions. The incubation period lasts from 14 to 20 days, and an individual is infectious from 1 to 2 days prior to development of skin lesions until all lesions have crusted [31]. As with HSV, VZV can be diagnosed via multiple tests, but PCR from a swab of a lesion is now commonly used.
Primary infection with VZV manifests as varicella (chickenpox), which presents with scattered vesicles on an erythematous base, resembling “dewdrops on rose petals.” Fever and influenza-like symptoms may occur. The cutaneous eruption and any systemic symptoms typically will self-resolve in immunocompetent hosts. Reactivation of the virus causes herpes zoster (shingles), presenting as a tender vesicular eruption in a dermatomal distribution (localized disease) (Fig. 10.2 ). A prodrome of itching, tingling, or burning is frequently reported in the involved dermatome. If the initial dermatomal VZV then disseminates, a workup for immunosuppression should be initiated, as intact immune systems generally contain the infection to one dermatome [26]. VZV is considered disseminated if cutaneous lesions cross three contiguous dermatomes, or 20 or more vesicles appear beyond the affected dermatome. Systemic involvement, including pneumonitis, meningoencephalitis, and hepatitis, as well as gastrointestinal tract and ocular involvement, can also develop following the cutaneous eruption. Recurrent primary varicella, defined as one or more episodes of disseminated VZV without an initial zosteriform distribution, has been reported in patients with HIV, leukemia, and lymphoma [32]. VZV may present atypically as hyperkeratotic, pustular, purpuric, or even ulcerated and necrotic lesions in immunosuppressed patients. Finally, chronic herpes zoster, lasting longer than 1 month, has been reported with advanced HIV, but less frequently in patients undergoing cancer chemotherapy or in posttransplant patients on immunosuppressive therapy [33]. These forms of zoster cause significant morbidity and even mortality in these populations.
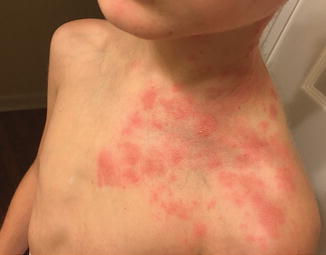

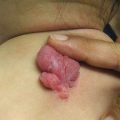
Fig. 10.2
Herpes zoster in a hematopoietic stem cell transplant recipient (image courtesy of Marissa J. Perman, MD)
Parenteral antiviral therapy should be initiated in immunosuppressed patients diagnosed with VZV infection. First-line treatment is IV acyclovir , with the goal of preventing progression to disseminated disease. Acyclovir resistance is less common in VZV than in HSV; however foscarnet can be used as a second-line agent when resistance is suspected [34].
Epstein-Barr Virus (EBV/HHV-4)
EBV is transmissible via contact with infected body fluid, including saliva, breast milk, and genital secretions. Incidence of primary EBV infection peaks from 1–6 to 14–20 years of age, and reactivation can occur with immunosuppression. EBV may be causative in multiple malignancies, including B-cell lymphoma and nasopharyngeal carcinoma. Natural killer/T-cell lymphoma (NKTL) is an aggressive lymphoma linked to EBV that classically presents with ulceration and necrosis involving the nose. It is more common in East Asian and Latin American patients. Immunosuppression is rarely reported to be an associated risk factor, and can result in atypical or severe disease [35].
EBV is also implicated in lymphoproliferative disorders such as posttransplant lymphoproliferative disorder (PTLD) . PTLD typically occurs 1–2 years following organ transplant with incidence varying based on organ type and immunosuppressive regimen [36]. Approximately 5% of cases will have cutaneous manifestations [36]. The cutaneous presentation of PTLD classically is that of indurated, violaceous papules and plaques; however, its appearance can range from a localized erythematous eruption to diffuse subcutaneous plaques, nodules, or masses [37]. See Chap. 11 for further discussion of PTLD.
EBV can also present as chronic ulceration of the cutaneous or mucous surfaces in immunosuppressed patients (mostly described in adults). PCR from an active lesion can demonstrate the virus, and withdrawal of immunosuppression can lead to spontaneous regression [38, 39]. Chronic ulcerations can be caused by many agents, but when considering EBV as an etiology, CMV should be in the differential diagnosis.
Human Herpesvirus 8
HHV-8 , a DNA gamma-herpesvirus , is causative in Kaposi sarcoma (KS) , as well as lymphoproliferative disorders like multicentric Castleman’s disease and hemophagocytic lymphohistiocytosis (HLH) . The virus can infect several different cell types, including monocytes, B lymphocytes, and oral epithelial cells [40]. It is important to note that the incidence of KS rose dramatically with the HIV epidemic, suggesting that the retrovirus may provide a cofactor necessary for the progression of HHV-8 to KS. This association remains even among individuals with well-controlled HIV on retroviral therapy [40].
The diagnosis of KS should be histologically confirmed; thus skin biopsy is the gold standard when this virally mediated disease is suspected; serology for HHV-8 is not required.
KS is classified into one of four types, based primarily on host characteristics and disease course. The classical and endemic (African) types largely occur in immunocompetent hosts. The remaining categories occur in special populations: iatrogenic/transplant patients and individuals with HIV/AIDS (epidemic KS).
The clinical presentation of HHV-8 varies depending on the immunologic status of the host at the time of infection. Primary infection may be mild and nonspecific, or even asymptomatic, in immunocompetent children. On the other hand, severe disease characterized by fever, bone marrow failure with plasmacytosis, and rapid dissemination has been reported in children with HIV and posttransplant patients [41].
In children, epidemic KS is an aggressive disease with significant lymphatic involvement, as well as classic pink-purple cutaneous papules and plaques. Lesions can be painful or pruritic and may koebnerize. They may initially be mistaken for hematoma, purpura, bacillary angiomatosis, or even hemangioma when localized. Extracutaneous spread is common, with involvement of the oral mucosa, gastrointestinal tract, and lungs. Gastrointestinal involvement may present as melena, hematemesis, or hematochezia, while dyspnea, nonproductive cough, or hemoptysis signals pulmonary disease.
The clinical spectrum of KS in the transplant population is not well elucidated; both primary infection from HHV-8-infected donor grafts and viral reactivation may occur. Cases have been reported in both solid-organ- and bone marrow-transplant patients. Manifestations are similar to that of epidemic KS, although prognosis may be poorer; severity is likely related to baseline HHV-8 immunity, degree of immunosuppression, and, in solid-organ-transplant patients, organ type [42].
No established guidelines exist for the treatment of KS and therapy should be guided by an infectious disease expert, especially someone with experience treating children. For primary HHV-8 infection, supportive care is recommended in immunocompetent patients, while antivirals such as ganciclovir or valganciclovir are recommended for immunosuppressed populations [40]. In localized epidemic KS, treatment typically consists of therapy for underlying HIV, intralesional vincristine, or topical alitretinoin. For patients with systemic or refractory disease, systemic chemotherapy protocols are considered [43]. In immunosuppressed patients, including transplant patients, a typical treatment approach includes reduction and modification of the immunosuppressive regimen, such as including tacrolimus or sirolimus, agents with antitumorigenic properties [40].
Human Papilloma Virus (HPV)
HPV is the most common viral skin infection, and is the etiologic agent of both common and genital verrucae (warts) [44]. There are more than 120 subtypes of HPV, many of which carry an anatomic predilection. Transmission occurs through skin-to-skin contact or via contaminated surfaces or objects. HPV infections occur in both immunocompetent and immunocompromised patients; however prevalence is increased in patients with impaired cell-mediated immunity [45]. Diagnosis is typically made clinically, or histologically with an HPV immunostain.
HPV lesions can be chronic, extensive, or atypical in appearance in immunosuppressed patients. Additionally, they may be refractory to multiple treatment modalities in this population [46]. Predisposition to HPV infection has also been associated with several syndromes. Epidermodysplasia verruciformis, a genetic disease due to mutations in EVER1/EVER2, predisposes to HPV 5 and 8. The typical lesions are flat-topped verrucae that are skin colored or tan brown and are recalcitrant to therapy. These lesions must be closely monitored over time due to risk of development of squamous cell carcinoma in the field. Other genetic syndromes presenting with verrucae include warts, hypogammaglobulinemia, infections and myelokathexis syndrome (WHIM), warts, immunodeficiency, lymphedema, dysplasia (WILD) syndrome, and GATA-2 mutations [47].
In immunocompetent patients, lesions can self-resolve over years, but therapy can be much more challenging in the setting of immunodeficiency. Many therapies work in whole or part via immunomodulatory mechanisms (i.e., cryotherapy, salicylic acid, podophyllin, imiquimod) and so may not be successful in the immunocompromised host. Additional options for recalcitrant warts include topical cidofovir or 5-flurouracil, systemic agents such as oral cimetidine or retinoids, and photodynamic therapy [48, 49].
Molluscum Contagiosum
Molluscum contagiosum virus is a DNA poxvirus . Infection occurs commonly via skin-to-skin contact or through contact with contaminated surfaces or objects in children and as a sexually transmitted infection in adults. It has increased prevalence in immunocompromised patients, particularly those with HIV/AIDS [50]. Diagnosis is made clinically or histopathologically.
Clinically, molluscum contagiosum lesions appear as firm, skin-colored, pearly papules with central umbilication. In children, they have a predilection for the trunk, thighs, buttock, and face. Similar to HPV, molluscum can cause significant morbidity in immunocompromised hosts as lesions can become widespread and extensive, and can be refractory to treatment. In immunocompromised hosts, the differential diagnosis of molluscoid lesions includes cryptococcal infection, histoplasmosis, and penicilliosis [51]. Options for therapy include manual expression (such as curettage or extraction), cryotherapy, and topical agents including cantharidin and cidofovir, in addition to photodynamic therapy [49, 52].
Human Polyomavirus
The human polyomaviruses include six small DNA viruses that appear to be ubiquitous in the environment; however these only cause significant disease in immunocompromised hosts; other identified human polyoma viruses have not been identified in human disease to date. JC and BK are polyoma viruses that can cause rapidly progressive neurologic decline and nephropathy in immunosuppressed patients, such as those treated with TNF alpha-blocking agents [53, 54].
Two polyomaviruses have been associated with cutaneous tumors. Merkel cell polyomavirus is implicated in some Merkel cell carcinomas (MCC), a rare but aggressive tumor most common in white males over the age of 50. This classically presents as a rapidly expanding pink to violaceous papule or nodule on sun-exposed skin. HIV, organ transplant, and CLL have been identified as major risk factors [55, 56]. There are rare reports of this malignancy in the pediatric population [57].
The trichodysplasia-spinulosa polyomavirus (TSPyV) has recently been identified as the cause of trichodysplasia spinulosa [58–60]. This rare eruption appears as numerous folliculocentric papules and keratin spines referred to as spicules, most prominently over the nose, eyebrows, and ears, but can be found on other areas of the body as well. There may be associated thickening of the skin and alopecia of the eyebrows and scalp, resulting in leonine facies [61]. This appears to occur exclusively among immunocompromised hosts, and can become of significant cosmetic concern. Topical cidofovir has been shown to be an effective therapy [62].
Fungal Infections
Key Points
Invasive mold infections can start in the skin and spread rapidly.
Disseminated yeast infections may first present in the skin with widespread papules.
Fungal spores are ubiquitous in our environment. Some fungi colonize the skin (such as Malassezia yeasts), some are pathogens in normal hosts (dermatophytes and Candida species), and some are opportunistic fungi that are not typically pathogens in normal hosts. Cutaneous fungal infections can occur due to primary inoculation, dissemination, or infection of a preexisting wound.
Dermatophyte Infections
Dermatophyte infections such as those caused by Trichophyton and Microsporum may be more common in immunosuppressed patients such as those with AIDS [63]. They do not typically cause invasive, life-threatening disease, but can invade into the dermis leading to more exuberant papulopustular eruptions. Therapy is typically with systemic agents such as azole or allyl amine antifungals or griseofulvin [64].
Opportunistic Yeast Infections
Malassezia yeasts are colonizers of normal skin, but in immunosuppressed patients they can lead to cutaneous infections. Overgrowth of Malassezia can cause severe seborrheic dermatitis and folliculitis, especially in immunosuppressed patients. More rarely, Malassezia infections of indwelling catheters, especially in neonates and those receiving parenteral nutrition, can lead to septicemia [65, 66]. Diagnosis can be challenging, as the yeast needs to be grown on lipid-containing media [67]. Therapy can be initiated with amphotericin or systemic azole antifungals. Interestingly, the fungemia has been shown to spontaneously resolve with removal of the catheter and discontinuation of the lipid-containing nutrition in adults [68].
Colonization with Candida species is common in the gastrointestinal tract, as well as the perineum and oral cavity, and overgrowth can lead to infection [67]. Localized candidal infection in the mouth (thrush) can be a presenting sign for immunosuppression in children [69].
Candidemia is a very important cause of sepsis in immunosuppressed patients. Many different Candida species , including Candida albicans and C. glabrata , can cause infection in immunosuppressed patients [67, 70]. Cutaneous candidal infections usually manifest as red patches with scaling and peripheral pustules, especially in moist areas such as neck, axillary, and inguinal folds. Topical therapy with an azole antifungal or nystatin is usually sufficient for localized disease. Disseminated candidiasis in an immunocompromised host requires systemic treatment. Fever is common; disseminated candidiasis can also be associated with muscle pain, presumably due to yeast infection into the muscles [71].
Disseminated yeast infections also can occur due to Aspergillus , Trichosporon , Fusarium , and other yeasts [67]. These typically present in the skin with widespread, red-purple macules, and papules [72]. In addition, they can lead to pulmonary, renal, and hepatic disease [67]. While blood cultures may grow the fungus, a biopsy with direct histopathologic visualization, as well as culture, may yield a faster result. A frozen section performed on a biopsy of a suspicious site aids in even more rapid diagnosis. Tissue stains such as periodic acid-Schiff (PAS) and Grocott methenamine-silver (GMS) can help visualize the fungi on histopathologic slide preparations.
Invasive Mold Disease
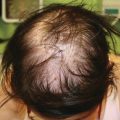
Mold will not typically live in the skin unless a patient is immunosuppressed. Mold infections can be rapidly fatal in immunosuppressed patients. The skin is a common portal of entry for fungal spores. Spores of fungi such as Aspergillus and Zygomyces are frequently in the air and take advantage of breaks in the skin or occlusion with tape or bandages. If an opportunistic fungal infection appears first in the skin and is recognized early, it may be cured before it disseminates. Disseminated disease that starts elsewhere in the body, such as in the lungs, may also appear in the skin early in its course. Proper evaluation of skin lesions can again lead to early diagnosis.
Aspergillus infection is a common cause of serious morbidity and mortality in immunosuppressed patients [73]. After the lungs, the skin is the second most common site for Aspergillus infection [73]. Aspergillus can present with papules, eschars, ulcerations (especially at sites of trauma), or pustules and plaques due to infiltration of the hair follicles. In one study, over half of patients’ skin disease was localized [74]. In addition to patients undergoing chemotherapy or bone marrow transplantation, extremely low-birth-weight infants are at risk for Aspergillus infection [75]. Tape or other occlusion can cause the fungus to proliferate (Fig. 10.3 ). Diagnosis of invasive Aspergillosis can be aided by the galactomannan blood test [76].